Addition of Grignard reagents to ketones to give tertiary alcohols
Description: When a ketone is treated with a Grignard reagent, a new C–C bond is formed at the carbonyl carbon. Subsequent addition of acid will then give the alcohol.
The rest of this page is available to MOC Members only.
To get access to this page, plus over 2500 quizzes, the Reaction Encyclopedia, Org 1 / Org 2 summary sheets, and flashcards, sign up here for only 30 cents/ day!
Real-Life Examples:
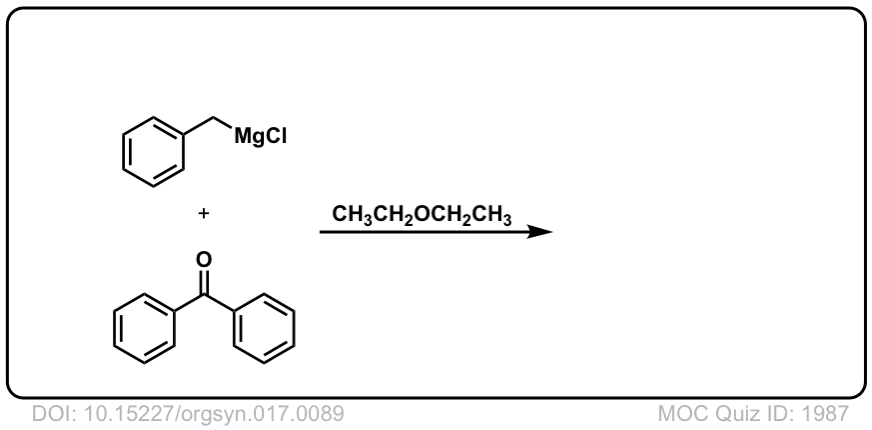
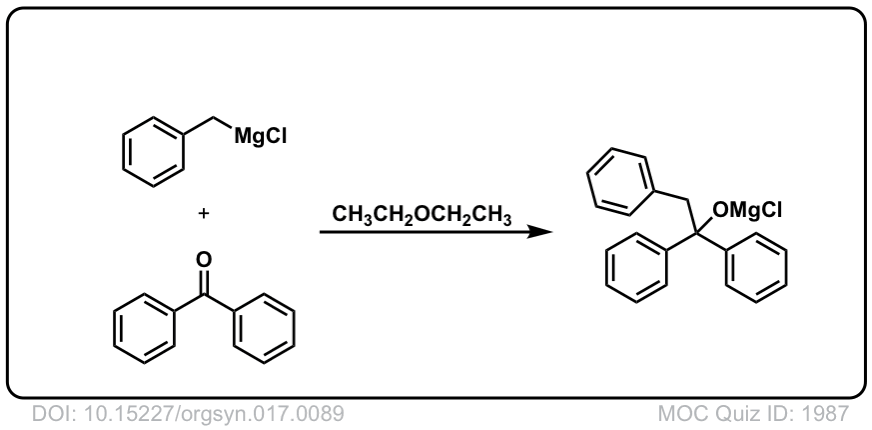
Org. Synth. 1937, 17, 89
DOI Link: 10.15227/orgsyn.017.0089
 Click to Flip
Click to Flip
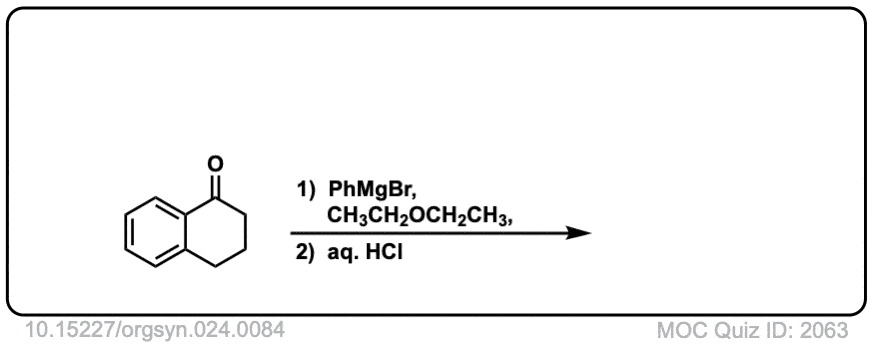
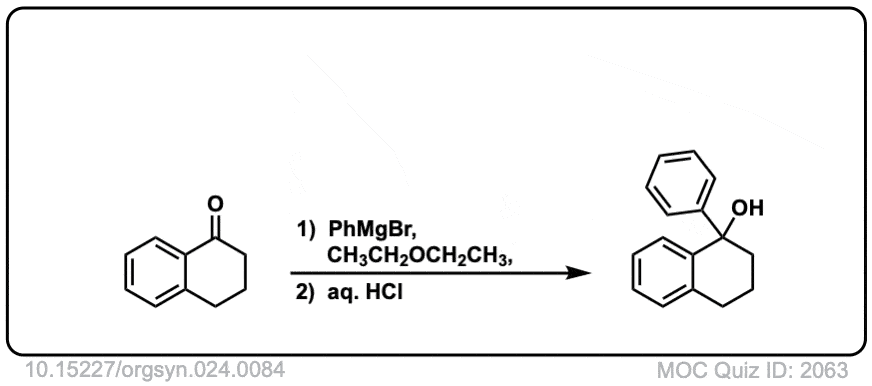
Org. Synth. 1944, 24, 84
DOI Link: 10.15227/orgsyn.024.0084
 Click to Flip
Click to Flip
Hello, in the fifth example, why doesn’t the alkene react with the acid in the second step?
Hi! In this case the H3O+ is a quench for a Grignard, which usually involves fairly mild aqueous acid (e.g. 1M HCl) at room temperature which is generally not strong enough to react with alkenes.
Some instructors are very picky about the H3O+ step doing exactly as you describe, so I will often specify “mild acidic workup” to differentiate. Thanks for the question!
Question! In the 3rd flip/quiz example, you have 9 carbons to work with and end up with 10. How/why? Thanks!
You are correct. Fixed! Thank you!
Confused about Example 4. Grignard is not on the final product, why is this?
That’s because #4 is a trick question! Acid base reactions are fast, relative to Grignard addition – the Grignard reacts with the carboxylic acid in an acid-base reaction, and the carboxylic acid is later protonated with acid workup. Very common trick question!
Hmm… Does Grignard react with hydrogen of alpha carbon?? Because that hydrogens are acidic, isn’t it??
Addition is faster than deprotonation. The C-H is only really acidic when aligned at 90° to the carbonyl, and deprotonation also involves considerable reorganization of atomic geometry (see: principle of least motion). For non-hindered ketones, addition is considerably faster. This can change for sterically hindered ketones, however…
Hi James,
Is there a rule for stereochemistry when adding a grignard? For instance, in example 1 and 2, would the stereocenter default to R or S?
“Conservation of optical inactivity”. If there’s no chiral influence on a reaction, the product of an achiral starting material with an achiral reagent will result in an optically inactive product. A racemic mixture of enantiomers, in other words.