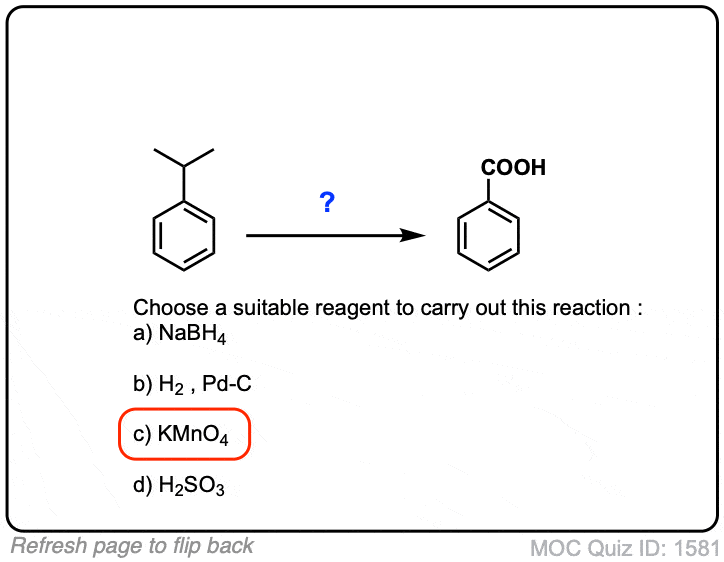
Oxidation of aromatic alkanes with KMnO4 to give carboxylic acids
Description: Treatment of an alkylbenzene with potassium permanganate results in oxidation to give the benzoic acid.
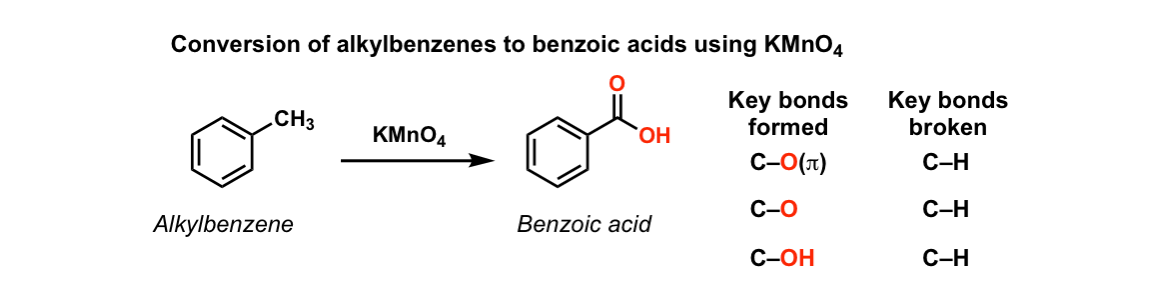
Notes: The position directly adjacent to an aromatic group is called the “benzylic” position.
The reaction only works if there is a hydrogen attached to the carbon.
Examples:
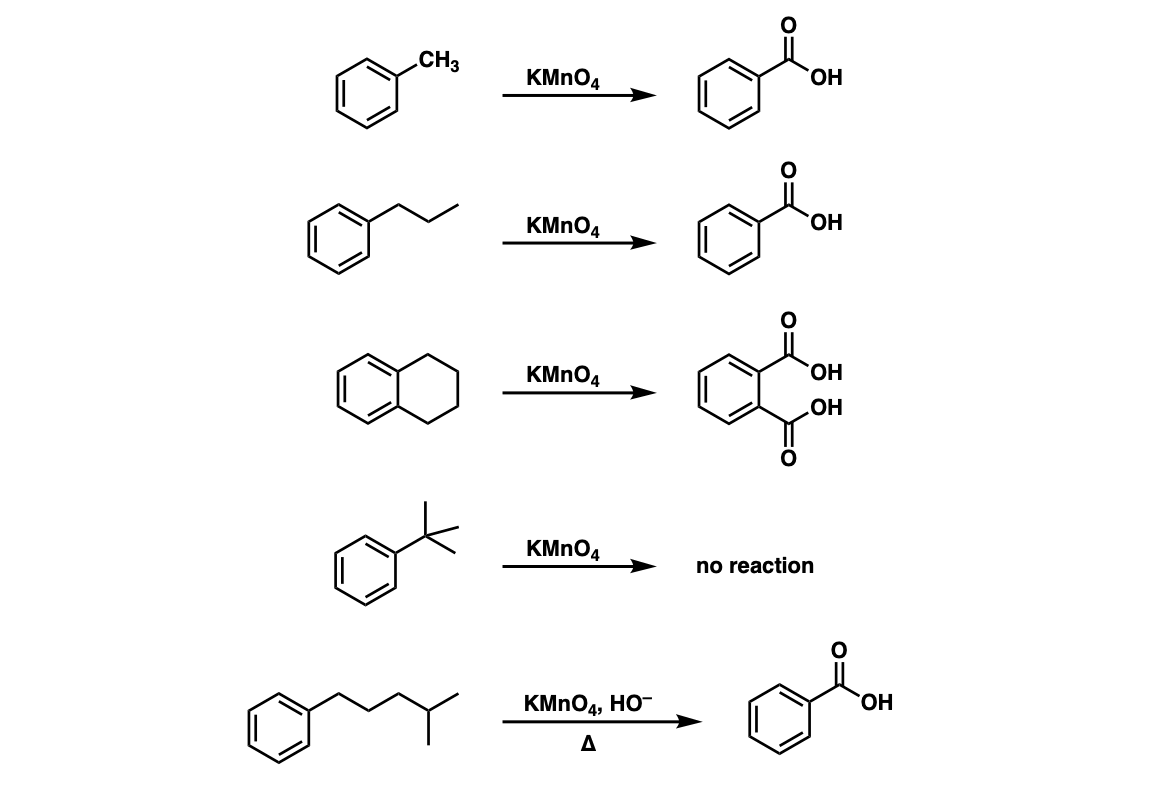
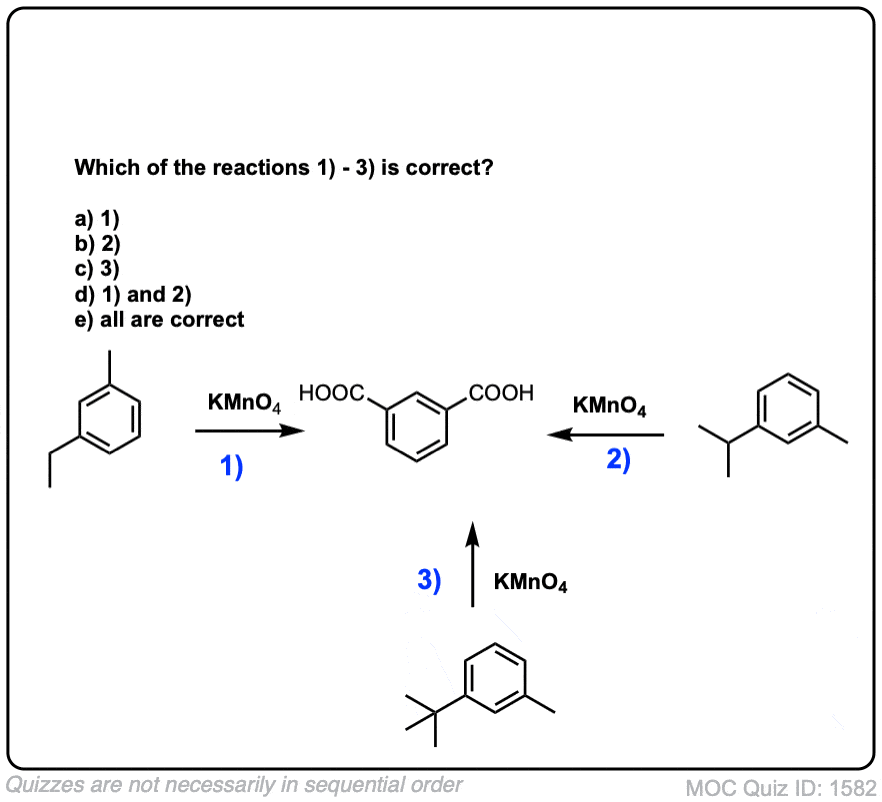
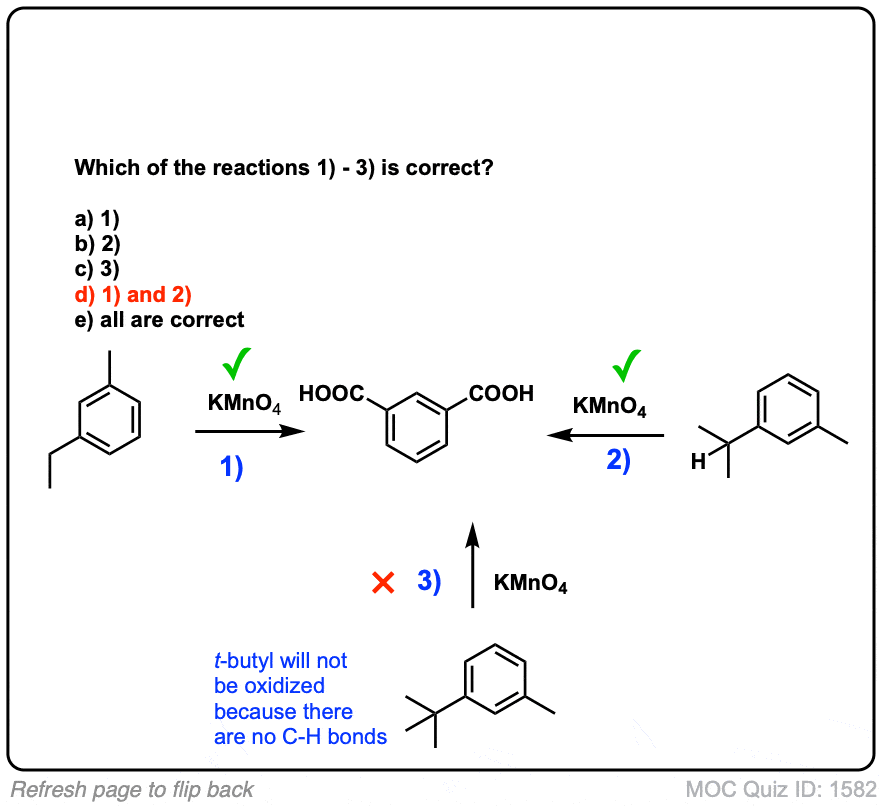
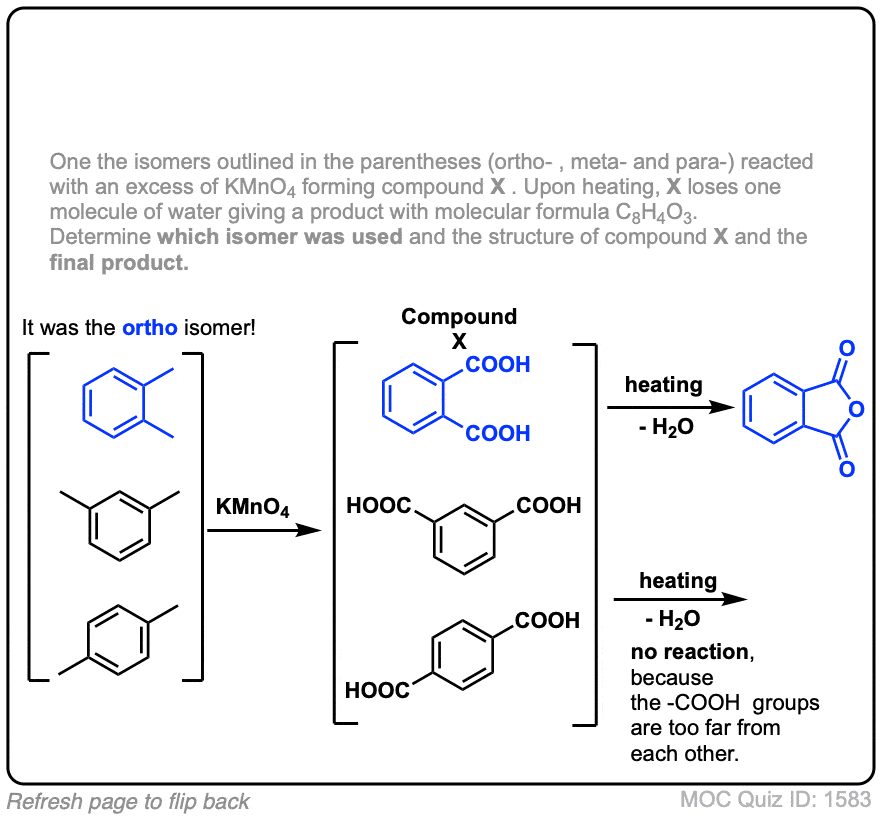
Notes: Note that in example 2 the extra carbons are cleaved to give the same product as in example 1. And in example 3, two benzoic acids are formed. Finally, when no hydrogens are present on the benzylic carbon, no reaction occurs (example 4).
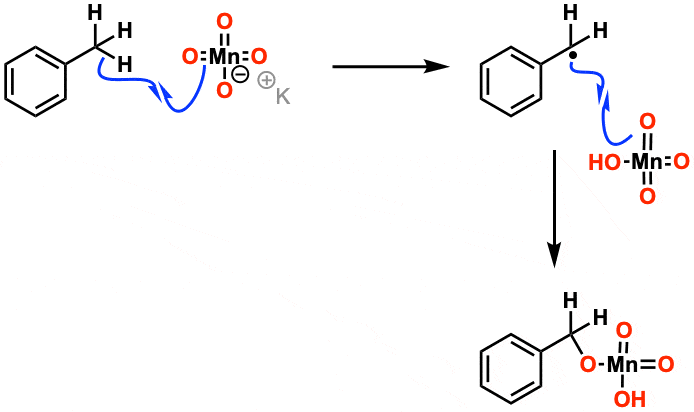
Mechanism: For the purposes of Org 1/ Org 2 the mechanism isn’t considered that important. Manganese acts in mysterious ways. It’s thought that the first step is removal of a hydrogen by one of the oxygens on MnO4(–) in a free-radical reaction. Beyond that, it gets complicated.
Quiz Yourself!
 Click to Flip
Click to Flip
 Click to Flip
Click to Flip
 Click to Flip
Click to Flip
(Advanced) References and Further Reading
For some examples “in the wild”, see the following.
- Mechanistic studies supporting an initial C-H abstraction. J. Am. Chem. Soc. 1963, 85, 3487.
- Another mechanistic study with tertiary benzylic carbon. J. Am. Chem. Soc. 1970 92 329
- Oxidation of various hydrocarbons with KMnO4 using trifluoroacetic acid: Can. J. Chem. 1978, 56, 1273
Real-Life Examples:
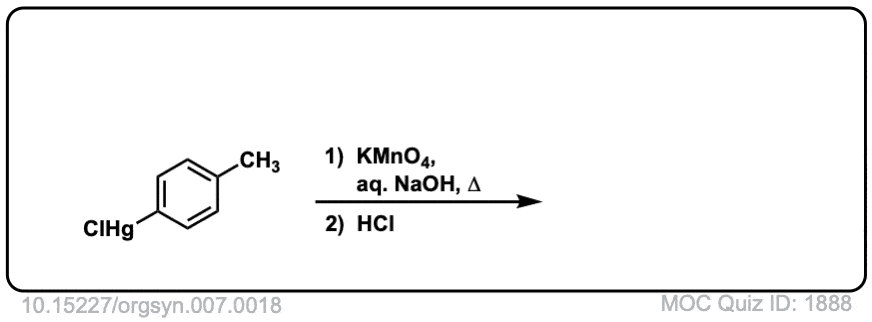
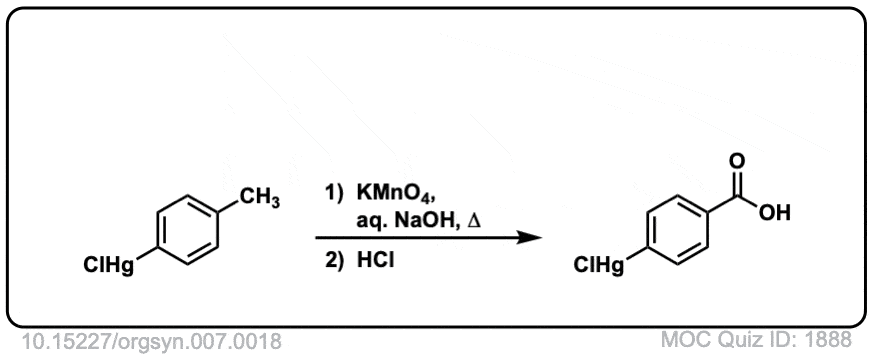
Org. Synth. 1927, 7, 18
DOI Link: 10.15227/orgsyn.007.0018
 Click to Flip
Click to Flip
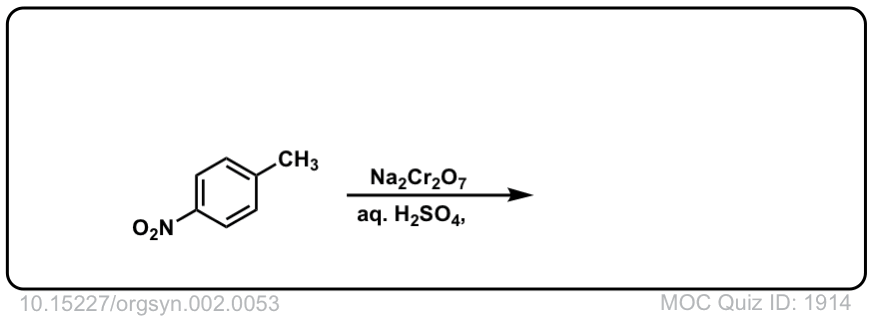
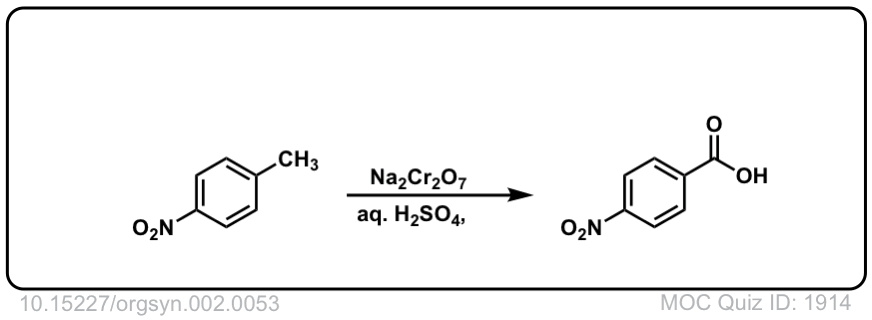
Org. Synth. 1922, 2, 53
DOI Link: 10.15227/orgsyn.002.0053
 Click to Flip
Click to Flip
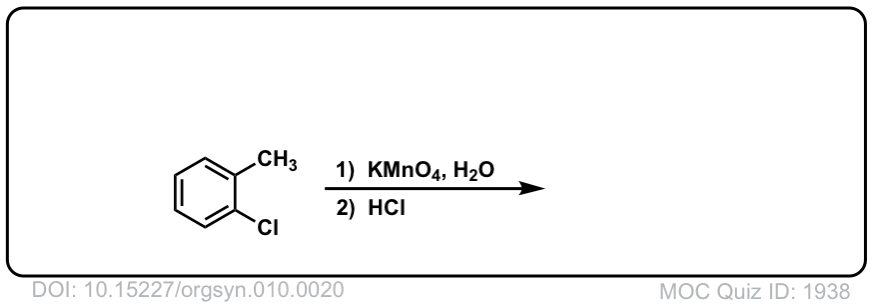
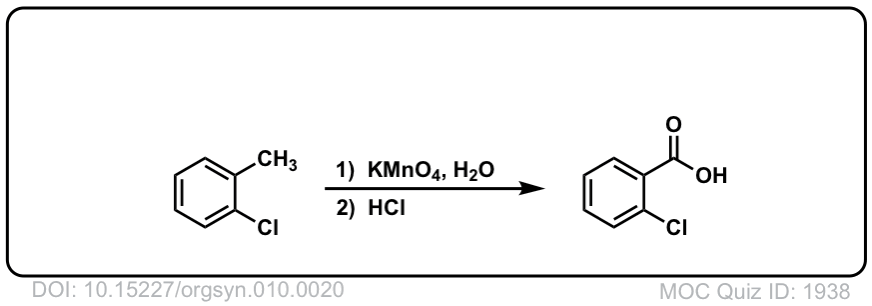
Org. Synth. 1930, 10, 20
DOI Link: 10.15227/orgsyn.010.0020
 Click to Flip
Click to Flip
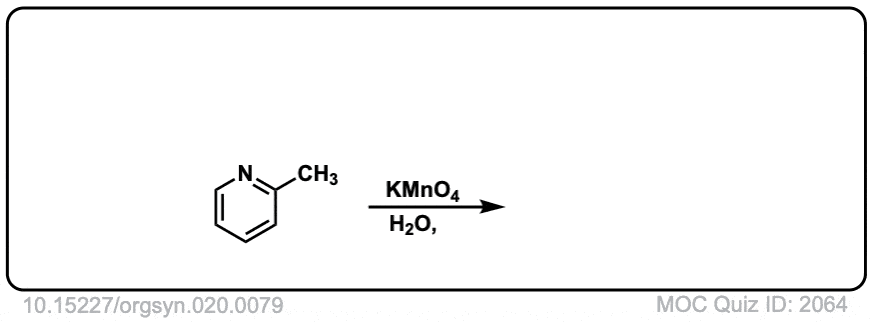
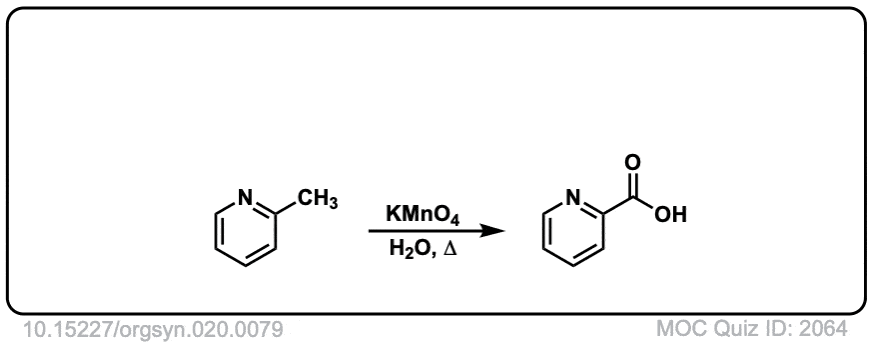
Org. Synth. 1940, 20, 79
DOI Link: 10.15227/orgsyn.020.0079
 Click to Flip
Click to Flip
Hello,
I recently did an experiment to test the reactions between Hexane, Hexene, Cyclohexane with
1. KMnO4 solution mixed with Sulphuric acid
2. Bromine water
The results were not as expected.
Hexane – KMnO4 decolourised
Hexene – KMnO4 did not decolourise
Cyclohexane – KMnO4 decolourised
Hexane + Bromine – No reaction (Which is correct)
Hexene + Bromine – Reaction was incomplete, it decolourised slightly to a light yellow
Cylohexane + Bromine – Decolourised with 2 layers formed, bottom layer being slightly light brownish
Hope you can explain why. Errors in labelling has been ruled out
Not being your TA, and not being familiar with exactly what you did in the lab, I can’t really comment, except to say that if you are observing a reaction between cyclohexane and bromine water, you have either discovered something miraculous that nobody else has before, or you made a mistake somewhere along the way.
Would 3-phenylprop-1-en treated with KMnO4 and heat create benzoic acid or would it just cleavage double bond and reaction would end here becaouse of no hydrogen atoms?
It would eventually go to benzoic acid.
Hi, is it possible that during the KMnO4 oxidation of styrene one of the products besides the benzoic acid and styrene glicol could be phenylacetic acid?
Not in any useful quantity.
If you want phenylacetic acid from styrene, one way to do it would be to perform hydroboration-oxidation to get phenylethanol. Then oxidize the primary alcohol up to the carboxylic acid using an oxidant like chromic acid or similar.
What will happen if I treat benzyl alcohol with Kmno4? Will I get phenylethanoic acid if not how to obtain phenylethanoic acid from benzyl alcohol
It will be oxidized to the carboxylic acid.
How would Styrene behave under such conditions? I came across a question in my test in which styrene reacted with KMnO4, I marked the option with vicinal diol, turns out I was wrong.
Depends on conditions. Cold, dilute KMnO4 with styrene would give a vicinal diol. But as soon as the reaction mixture is heated, it would undergo oxidative cleavage to form benzaldehyde, which would then oxidize further to benzoic acid.
If I use distilled water and THF to dissolve a mix of KMnO4 and HNO3, is THF would be oxidized by KMnO4?
One concern with THF is that strongly acidic conditions will open the ring. Probably not the most robust solvent under those conditions.
Phenol can be directly converted to benzylic acid by treating it with kmno4?
Absolutely not.
What if salicylaldehyde reacts with KMnO4
Probably a big mess, since the phenol portion will oxidize to give quinones, etc. If you want the carboxylic acid, there are better methods available.
What will happen when mesitylene is treated with KMnO4
Can be oxidized all the way to trimesic acid.
From the following reference (open access) – https://cdnsciencepub.com/doi/pdf/10.1139/v89-211
. A series of alkyl-aromatic compounds has recently
been oxidized by potassium permanganate in the presence of a
phase-transfer catalyst (8-12). In this way toluene, stilbene,
p-xylene, mesitylene, and durene were oxidized with potassium
permanganate in a two-phase system (hydrocarbon/water) containing cetyltrimethylammonium bromide (CTAB) in 1 : 1 :3.3 X
1OP3:138 molar ratio (ArH:KMn04:CTAB:H,0) at 70-8VC,
yielding the corresponding benzenecarboxylic acids, i.e., benzoic (93%), terephthalic (95%), trimesic (90%), pyromellitic
(80%), and benzoic (84%) acids, respectively (8).
Hi James, Could you please show the mechanism for the oxidation of graphite with KMnO4 in concentrated sulfuric acid
(2,2-dimethylpropyl)benzene react with kmno4 ,does it form benzoic acid?
There is no C-H bond on the benzylic carbon.
Hi James.
Can KMnO4 be used to to produce benzoic acid from cumene in an acidic environment? I’m using acetic acid as a solvent. Also, what te.perature and time should be required?
Hi – yes, I have several references you might find useful that are examples of oxidations of primary and secondary alkyl aromatics.
They are fairly old but they work. This one covers cumene (isopropylbenzene) specifically.
https://pubs.rsc.org/en/content/articlelanding/1955/jr/jr9550004186
https://pubs.rsc.org/en/content/articlelanding/1943/JR/JR9430000144
Its said that for converting toluene to benzoic acid the most suitable reagent is alcoholic KMnO4 …. though we know full well that acidic KMnO4 or alkaline KMnO4 are good oxidising agents . Can anyone say if KMnO4 oxidise alcohol to ethanoic acid then is it not possible that the yield of benzoic acid obtained will be less than if acidic KMnO4 was used> ..since some of the KMnO4 is already used to oxidise the ethanol ? or is it because EtOH promotes radical reactions …. which appears not too justifying.
What happens when 3 phenyl propanal is oxidised with kmno4? Does it convert to 3 phenyl propanoic acid or benzoic acid? (I think it would the first one).
At low temperatures, the carboxylic acid. If heated with excess KMnO4, the full oxidation to benzoic acid will occur.
I had a question. If the presence of a handheld hydrogen is necessary, then why does acetophenone get oxidized to Benzoic acid upon subsequent oxidation?
Acetophenone is in equilibrium with its enol tautomer, which reacts with KMnO4. Benzophenone would not react.
Will the the same reaction occur if we take Naphthalene ?
If you heat naphthalene up enough with KMnO4, you will get phthalic acid. https://en.wikipedia.org/wiki/Phthalic_acid
Hi, I have a question about the second example, why the methyl group in m-tert-butyl toluene doesn’t oxidize with Kmno4?
I’m not seeing m-tert-butyl toluene in the second example, I’m just seeing t-butyl benzene.
Thank you, Sir.
Moreover, it is then just phenol and aryl amines that decolourise Baeyer’s reagent.
I actually don’t understand why benzene and aryl chlorides do not decolourise it but phenol does.
Maybe phenol reacts in a different way unlike the alkenes.
I would be grateful if you enlightened me a bit.
I have an off track doubt. What happens if we add Baeyer’s reagent to an aryl halide like chlorobenzene?
Is there any reaction?
No reaction between cold, basic, dilute KMnO4 and chlorobenzene.
Could the KMnO4 oxidize the benzene ring itself?
Generally not under normal conditions, though anything is possible with enough KMnO4 and heat.
Can KMnO4 oxidize CARDANOL to Hydroxyl benzoic acid?
I had to look up the structure of cardanol. With a phenol on the ring, you’re likely going to get quinone formation and a total mess. Find another way to do it.
would it still oxidize to benzoic acid if the benzylic position is quartairy but one of the substitutes of the benzylic position is an amine?
I don’t know what it would make if there was an amine; it’s likely going to be a mess.
What happens if diphenyl methane is used as reactant? Do 2 molecules of benzoic acid form, or 1 benzoic acid and 1 benzene. Do products differ if oxidant is L.R first and then in excess?
I don’t know. You’d get benzophenone for sure. After that, I’d think that given mild enough conditions you’d be able to stop it there.
What is the colour change for lets say methylbenzene to benzoic acid?
Color change is a part-per-million phenomenon that is often not diagnostic. Methylbenzene (toluene) is colorless, and benzoic acid is also colorless. But trace impurities can lead to color.
If benzene side chain alkene present instead of alkane what will happen
Styrene? Likely dihydroxylation first, and then oxidative cleavage, and you’ll get benzaldehyde, which should oxidize further to benzoic acid.
will it work if there’s a ketone attached to the benzylic carbon followed by an R group? So C6H5COCH3 for example
Not sure. If the enol is formed, then that will certainly react with KMnO4.
Can kmno4 oxidize acetophenone to benzoic acid?
The alpha position of acetophenone is reasonably susceptible to radical C-H abstraction. Given enough heating I would not be surprised to see oxidation.
Does it work if instead of a carbon chain, there is an alkyl halide on the benzene ring?
Such as CH2Cl ? That would be fine, it should still oxidize as it will still form a resonance stabilized free radical.
Hi there… How about this compound.. (C6H5)-CH2C(CH3)2OH?.. Could it be oxidised to benzoic acid as well? Even though it is a tertiary alcohols?
It will oxidize to benzoic acid because it has a benzylic C-H bond.
What about oxidation of secondary alkyl benzene?
Above given examples are only primary and tertiary alkyl benzene
Still works.
Hi James, what if side chain have one double bond in example 2. Will the main product be same or change??
The double bond will be dihydroxylated by KMnO4. At low temperatures with base this can be isolated as a diol. With heating or acid oxidative cleavage will occur to give an aldehyde which can be further oxidized to give a carboxylic acid.
Why not K2Cr2O7 not used in oxidation of toluene?
With acid, as chromic acid H2CrO4, it can be used in the oxidation of toluene and other aliphatic compounds. See here. Tetrahedron 1941, 20, 1151 . https://www.sciencedirect.com/science/article/pii/S0040402001989823
if (C6H5)CH2CH2OH reacted with H+/KMnO4 —>>
what product formed???
benzoic acid or 2-phenylethoic acid ???
Benzoic acid
Why does styrene react with KMnO4 to give benzaldehyde? Why not benzoic acid?
Benzaldehyde would be the first product formed after oxidative cleavage of the glycol. It wouldn’t be difficult for benzaldehyde to be further oxidized to benzoic acid.
I have two methyl groups at pyrazole ring at 1,3 position. I want to convert both groups to CA. I tried so many times with KMNO4 +water or KMNO4+ pyridine or KMNO4+NaOH. but I could not get acid groups. Please hewlp me or suggest me some other route.
It’s probably hitting the pyrazole nitrogens, forming an N-oxide. I would suggest doing benzylic bromination or similar reaction first, followed by conversion to alcohol and then mild oxidation to the carboxylic acd.
If the compound we treat with KMnO4 is a group like -CH2COCH3 Would that oxidize by the reagent ? Since this substitute has alpha carbon but also this is a ketone group
If the compound we treat with KMnO4 is a group like R’-CH2COCH3 (R’=benzene) Would that oxidize by the reagent ? Since this substitute has alpha carbon but also this is a ketone group
Yes, it would definitely be oxidized to benzoic acid.
I believe if you use alkaline KMnO4, then you will not get the observation “purple solution is decolourized”.. instead you might get “purple solution produces brown ppt”.
Can any one have any idaa what is brown ppt??
MnO2 and other manganese salt crap. Can’t be more precise than that.
Where can you draw these chemical equations?
ChemDraw.
Can methanoic acid be oxidized to carbondioxide using acidified KMnO4??
You mean formic acid? Sure.
What about activated benzene ring with KMnO4 and heat?
In my notes o cresol decomposes
So will every activated ring be decomposed ?
Anything with an OH directly attached to the ring will be oxidized to a quinone and from there it will produce a polymerized mess.
For the first example, is there a way to add the carboxylic acid group to the structure so that there is a carbon between the benzene ring and the carbonyl carbon? Thanks!
Not using this reaction, no.
does this only happen in acidic and high temperature condition?
Yes, it’s generally a reaction that requires heat. Although… there are some variants of organic-soluble permanganate salts that can oxidize once to give alcohols and not give further reactions. See for example, https://science.sciencemag.org/content/324/5924/238 (full disclosure: I am an author).
Why doesnt the KMnO4 attack the pi bonds on the aromatic
Aromatic rings are generally quite resistant to electrophilic attack.
Whats the mechanism of action of the oxidation reaction taking place how the mno4 ion attack on the alkyl benzene and form cooh grp. Can u show it structurally.
See the references attached.
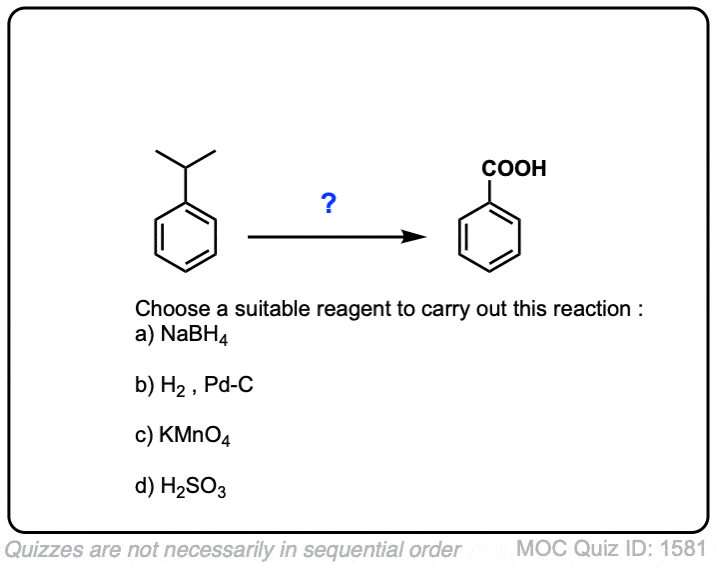
So does KMnO4 also turn isopropylbenzene to a carboxylic acid as well? So would that be benzoic acid?
Yes, that’s correct!
Does C6H5CHO (BENZALDYHYDE) reacts with H+/KMnO4 giving Benzoic acid?
Oh, absolutely. Oxidation of aldehydes to carboxylic acids is extremely easy. If you leave benzaldehyde out long enough, air will oxidize it to the carboxylic acid. Using KMnO4 is like using a thermonuclear bomb to kill a moth.
Anyone know the reagents and conditions for the oxidation of benzaldehyde to benzoic acid?
Just leave it out on the bench for a few days, it will air-oxidize.
Hey/ KMnO4 will oxidise the benzene itself like ozone. If not why decolourisation of KMnO4 occurres
KMnO4 will not easily react with the aromatic ring, nor will ozone.
Will a similar reaction occur if the ring is partly saturated (e.g. cyclohexene instead of benzene)?
In that case, the double bond of cyclohexene will be attacked. No reaction on the sidechain will occur because no resonance-stabilized benzylic radical can be formed.
Hey, Would this reaction be given by cumene?will it get oxidised to benzoic acid?
Yes, it absolutely would.
Can xylene isomers be converted to toluene with potassium permanganate?
No, you’d get dicarboxylic acids.
Can benzoic acid be further oxidized into an alcohol c6h5ch2OH
That’s not an oxidation, that’s a reduction.
Hey,
If the benzene is attached to a cyclohexene and you add this molecule to kmno4 for 2 hours at room temperature, what is the product? Do you get a ketone at the benzyllic position with a carboxylic acid?
Thanks
Does KMn04 has an effect on Ester bond?
Generally, no, although it certainly could oxidize the carbon adjacent to the oxygen.
Will acetophenone show this reaction? It has no hydrogen but i guess it still shows this reaction.
In the presence of acid, acetophenone will exist as its enol form. The enol form, which contains an alkene, WILL react with KMnO4 to give the diol, and then it’s off to the races.
If there is 10 carbon in second example what would be the reaction and product ? Dose that reaction is too hard to do?
Still benzoic acid. Not significantly harder than ethylbenzene. Still goes through a benzylic radical.
HI James
Do you know how the oxidizing power (or ability) of KMnO4 compares to microbial enzymes for degrading biomolecules found in plant material or soil organic matter?
My understanding is that KMnO4 is less “hot” than some of the iron oxo compounds that are found in liver enzymes, although their chemistry is quite similar.
What about if you had (ethoxymethyl)benzene and attempted this oxidation? Would you oxidize the benzylic carbon to a carbonyl and keep the ether linkage, or would you get a carboxylic acid as is outlined above?
Thanks.
Great question. You’d likely oxidize the two C-H bonds on the benzylic carbon to C-O, which would give you an ester, and under the typical reaction conditions (aqueous acid, heat) , you’d probably hydrolyze it. My guess is that the CH2 group on the ethoxy linkage would oxidize as well, since the reaction proceeds through a free radical mechanism and radicals on carbons directly attached to O are stabilized.
What is the effect of substituents attached to benzene ring is dere any ?
Should be pretty minimal, although you’d want to avoid any substituents that are easily oxidized, like NH2 or SR.
I was under the impression that heat was a necessary catalyst.
I wouldn’t say that heat is a “catalyst” but the reaction is definitely helped by heat, yes.
Hw about there is a alcohol group attached to the alkyl side chain ? The reaction still same?
It would get oxidized to a ketone.
What will be the result if any organic compound undergo complete oxidation?
COMPLETE oxidation?? CO2 !
why is conc. HCl added ?
Protonate the carboxylic acid at the end.
Sorry. The bezylic position has 3 OH GROUPS
A carbon with three OH groups? How is that stable?
Is the oxidation of side chain directly attached to cyclo hexane possible ?
Not using these conditions, because the cyclohexane does not afford resonance stabilization.
Hi James if the benzene ring has a carbon which has 3 OH groups attached to it will it get oxidised to benzoic acid?
I the beryllium carbon odes not have a hydrogen?
hi. i need to know the mechanism of the reaction of ethyl benzene with KMnO4…
actually i want to know why both methyl benzene and ethyl benzene give the same product…
thank you in advanced
See references at bottom of the page.
Would this also work with chromium trioxide as the oxidising agent ?
Thanks,
Parakh
thanks for the great info!
you mentioned that a benzylic H is necessary for this reaction to occur. However i read in Solomons that even PhCOCh2Ch3 reacts and gives Ph COOH .how can this happen?
thanks again.
That’s an ester hydrolysis, not an oxidation.Sorry, saw an extra oxygen where there wasn’t one.Yes, hydrogens on carbons adjacent to carbonyls contain weaker C-H bonds than do normal alkanes, so this reaction is possible. They are weaker because of an effect known as “captodative stabilization”, where the free radical initially generated is stabilized by interaction with the orbitals of the carbonyl. I know this is a very technical sounding answer, but it’s not dealt with very much in introductory organic chemistry.
Isn’t PhCOCh2Ch3 an aromatic ketone?
Thanks for pointing out my error. Corrected above.
I believe also in this particular case, the enol double bond can be broken by KMnO4 as alkenes do, forming the same product.
Why catalysts work only at side chain carbon atom? Why not carbon-H bond of benzene ring?
Hi there.. just to confirm. so does that mean if the side chain is a ketone it wont get oxidised? because the adjacent carbon to the aromatic ring has no H. is that a fact?
If it’s an enolizable ketone, it will get oxidized.
How do we add COOH group while replacing benzylic hydrogen of say toluene?
Lots of ways. Benzylic bromination followed by NaCN followed by aqueous hydrolysis… benzylic bromination, metal halogen exchange, CO2 then acid…
I thought KMnO4 could be used to create double bonds? For example if I used NBS to attach a Bromine to the benzylic carbon, could I then used KMnO4 to create a double bond and remove the Bromine? Do I have my reactants mixed up?
Yeah, KMnO4’s not the one. You want KOtBu or some other good base.
Say there is a halogen attached to the benzene ring, will it remain while the alkyl chain is oxidized? Thank you for this helpful page!
yep! it will stay.
Do we use acidified or alkaline KMnO4? Or it doesn’t really matter?
Great website though! Helped me so much! People like you deserve happiness :’)
Hi Ashley.. I believe if you use alkaline KMnO4, then you will not get the observation “purple solution is decolourized”.. instead you might get “purple solution produces brown ppt”. Acidified KMnO4 is needed to give the “decolourized” observation.
BTW.. a BUG *THUMBS-UP* to those of you who manage this website. It is an EXCELLENT resource for Organic Chem, right till A Level !!! :)
Can you please tell me if KMnO4 will react with one of these, if not which one and the name please?
CH3-CH-OH-CH2-CH3
Cyclobutyl alcohol and methyl cycloprophy alcohol?
Under mild conditions, each of these would be oxidized to ketones, to start with. If any enolization starts to occur (acid would help with that) then the enol would be dihydroxylated and you’d end up with oxidative cleavage products.
Thanks for this great and very helpful website!!
my textbook says that reacting cyclopentene with KMnO4 will give you a di-ol…
That’s an alkene. Ocidation of alkanes gives carboxylic acid except oxidation with CrO2Cl2 wich gives an aldehyde. Oxidation of alkenes gives diols and oxidation of alkynes gives a dioc acid
Why is it preferred to use alkali when KMnO4 uses acid in the oxidation of alcohols?
Hello James,
Is it only KMnO4 has the ability to oxidise benzylic carbon to carboxylic acid, is there other oxidant capable of doing this?
Will this oxidation reaction also possible for allylic carbon?
Great question! Yes, in theory, KMnO4 is useful for such a task. However in practice, other reagents such as SeO2 (among many others) are typically used for this reaction. Google “allylic oxidation”.
K2Cr2O7 will do the job!!
With acid, yes.
Wouldn’t the methyl group be oxidised to methanoic acid as well?
Oh sorry I forgot that methanoic acid is further oxidised to carbon dioxide and water.
Since in the second example the second product is acetic acid, would the products of reaction n°3 be phtalic acid and oxalic acid?
Assuming excess KMnO4, it would probably eventually convert to oxalic acid and then 2 equivalents of CO2.
I want to know the mechanism of this reaction!
Are there any other ways to remove the methyl group where the mechanism is known? i.e. substitute it for a Br or F? and then add a carboxylic acid afterwards?
Thanks
If there is a halogen let’s say attached to our alkyl group will it form carboxylic acid ? and if there is an alkene not directly attached to the benzene but in the side chain will it form carboxylic acid?
Yes, as long as the carbon has at least one H in addition to the halogen, it will still form a caroboxylic acid – the chlorine will eventually get hydrolyzed. As far as the side chain is concerned, it will be completely cleaved until only the carboxylic acid attached to the ring remains.
What if potassium permanganate reacts with a Benzene (none substituted) will there be any product since KMnO4 usually racts with the Benzylic Hydrogen
Under normal conditions, no, there should be no reaction.
Explain example 3 again,please?
In this example both CH2 groups are cleaved to give carboxylic acids. The alkyl C-C bonds are cleaved in the process, resulting in breakage of the cyclohexane ring.
what other chemical that can be used to replace KMNO4??
CrO3 is also occasionally used for this reaction.
can i have the potassium dichromate oxidation mechanism of toluene followed by heating?
I suggest you check out the references at the bottom in “further reading”.
should the KMnO4 be acididic or alkaline?
is it possible to get the actual mechanism involed, using example 1
It’s a long sequence beginning with hydrogen abstraction and “rebound” of oxygen to carbon. There’s some uncertainty about the exact mechanism, so it hasn’t been shown.
Can Kmno4 oxidise methane as well?
If not, why?
Given enough heat, sure. It would give CO2. But so would plain old combustion.
what is the difference in the oxidation products of hexane,cyclohexane,xylene,benzene,benzylchloride,and methyl benzene by KMnO4? i cant understand the reactions
If I have a molecule like methylidene cyclopentane..does KMnO4 react to give a CA??
For methylidene cyclopentane, it would cleave the C=C bond, to give cyclopentanone and formic acid. Actually the formic acid would probably become CO2.
where can i find the reaction that you are talking about, above?
If I have a molecule like methylidene cyclopentane..does KMnO4 react to give a CA??
Does H2CrO4 have a similar mechanism/yield the same product?
Yes, I believe that H2CrO4 can also be used for this purpose. Not sure about the mechanism; in both cases, it’s fairly obscure.
You said the mechanism for the KMnO4 causing a carboxylic acid formation to the CH3 of the benzene is too complex for this site. Do you know where I can find the mechanism?
You might start with looking through March’s Advanced Organic Chemistry for this reaction and follow the reference from there. Essentially the first step is that KMnO4 removes a hydrogen from the benzylic position, forming a benzyl radical, and the oxygen then “rebounds” back to the carbon to form C-O. This repeats several times; the overall mechanism can go through several different pathways, but this is the essentials of it.
can u tell me the whole mechanism
I suggest you read the references at the bottom of the page.
In the second example, there is a three-carbon chain on the benzene, KMnO4 cleaves the chain leaving just one C (carboxylic acid). What is the other product formed then? Does the 2 carbon-chain cleaved off get oxidized too, so you’d have H3C-COOH? Or does it just stay as ethane or what? Essentially, does KMnO4 oxidize both sides of the benzylic hydrogens so it cleaves both sides into having a -COOH group on the cleaved side, or does KMnO4 only oxidize the side with the benzene to have a carboxylic acid?
Thanks for this great and very helpful website!
Yes, the other product would cleave as a carboxylic acid to give H3C-COOH although it’s quite possible that under these reaction conditions the alpha-position of the carboxylic acid would undergo further oxidation to HOOC-COOH, (oxalic acid) which would then probably oxidize to CO2.
In short, yes – KMnO4 should oxidize both sides.
If one carbon is getting cleaved from the molecule then I guess it should form HCOOH instead of CH3COOH (as mentioned by you guys above). And further if KMnO4 is in excess amount then HCOOH should probably get oxidised to CO2.
Correct me if I am wrong.
IAmAGeek, TA asked about the second example. The second example has a three carbon chain attached to the benzene ring. Therefore, two of those three carbons would be cleaved as an ethane chain which would then be oxidized to form H3C-COOH.
if you start with “propanyl-benzene”? you add the carboxylic acid group on the carbon closest to the ring.. once leaved of, youll have a piece if CH3-CH2 which will be oxidized to CH3COOH
Sure, but that will probably eventually oxidize to CO2 unless you’re careful.
Yes James is halfway correct only thing he missed is is a tertbutyl carboxylic acid
So we have tbutyly carboxylic acid ,oxalic acid (2 moles to be specific ) and CO2
The reactions skeletal mechanism can be seen by action of KMnO4 on each of benzenes double bond converting the ends to carboxylic acids , the upper part with the tertbutyl group is oxidized twice
show the mechansim for oxidation of toluene with kmno4
The first step is C-H abstraction by the Mn=O oxo bond, followed by internal return making a manganate ester. After that, it gets complicated.