Stereochemistry and Chirality
What’s a Racemic Mixture?
Last updated: July 5th, 2025 |
What’s A Racemic Mixture?
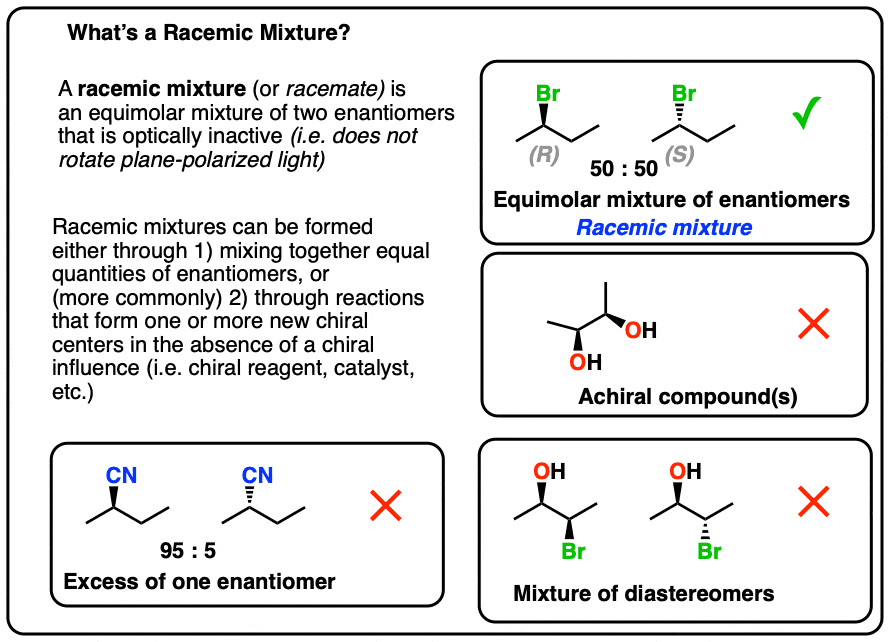
A racemic mixture is an equimolar mixture of two enantiomers that is optically inactive.
In this article we break down what a racemic mixture is, and give plenty of examples.
Table of Contents
- Definition of a Racemic Mixture
- “A Racemic Mixture Consists of Two Enantiomers…”
- “A Racemic Mixture Is Equimolar…”
- “A Racemic Mixture Is Optically Inactive…”
- Racemic Mixtures Through Combining Optically Pure Enantiomers
- How Do You Know If A Reaction Will Create A Racemic Mixture?
- Example #1: Addition of HBr To An Alkene
- Example #2: Free-Radical Addition of HBr
- Example #3: Alkene Hydrogenation
- Example #4: SN2 Of Alkyl Halides
- Example #5: SN1 Of Alkyl Halides
- Example #6: Pre-existing chirality example
- Example #7: Fun With cis-But-2-ene
- Example #8: Fun With trans-But-2-ene
- Example #9: A Chiral Reagent
- Conclusion
- Notes
- Quiz Yourself!
1. Definition of a Racemic Mixture
According to IUPAC*, a racemic mixture (a.k.a. a “racemate”) is
- an equimolar mixture of
- two enantiomers that is
- optically inactive.
All three conditions have to be met in order for a mixture to be classified as “racemic”.
Mind you, in 99.99% of cases you will likely encounter, satisfying the first two conditions will satisfy the third condition. [Note 1]
*Note that IUPAC doesn’t like the term racemic mixture, preferring racemate instead. We will use racemic mixture because it continues to be taught in introductory classes.

You will often see a racemic mixture denoted by (+/-) or (less often) by dl- or rac- .
This is used to indicate equal amounts of the (+) (dextrorotatory, rotates plane-polarized light clockwise) and (-) (levorotatory, rotates plane-polarized light counterclockwise) enantiomers.
[See: Optical Purity and Enantiomeric Excess]
2. “A Racemic Mixture Consists of Two Enantiomers…” ?
Let’s cover “enantiomers” first, because then it will become more clear as to why “equimolar” is important.
When we say molecules are enantiomers, we’re describing a relationship between two molecules – in the same way that the words, “brothers” or “cousins” describe a relationship between people. I can be a brother to one person but a cousin to another; in turn, my cousin’s sister is my cousin.
[For more discussion on enantiomers (and on brothers) : see “Types of Isomers” ]
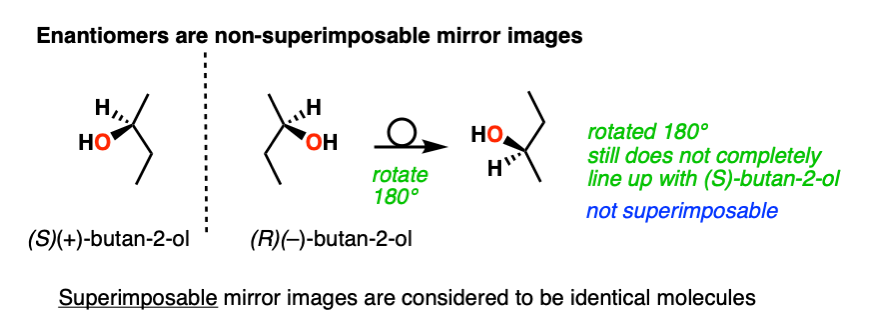
The term “enantiomer” is used to describe the relationship between two molecules that are non-superimposable mirror images of each other. The most common situation where this occurs is when a molecule contains a single chiral center. This causes the molecule to lack a plane of symmetry.
Why bother with this disclaimer about “non-superimposable” mirror images? That’s because the mirror image of an achiral molecule like ethane is just a rotated version of the same molecule and is therefore superimposable. As far as chemistry is concerned, two superimposable molecules are identical.
This is not the case for a chiral molecule, such as (R)-butan-2-ol and its enantiomer (S)-butan-2-ol.
The two are not superimposable on each other, and are therefore not the same molecule. They are enantiomers.
This isn’t unique to molecules, by the way. It can happen in LEGO too!
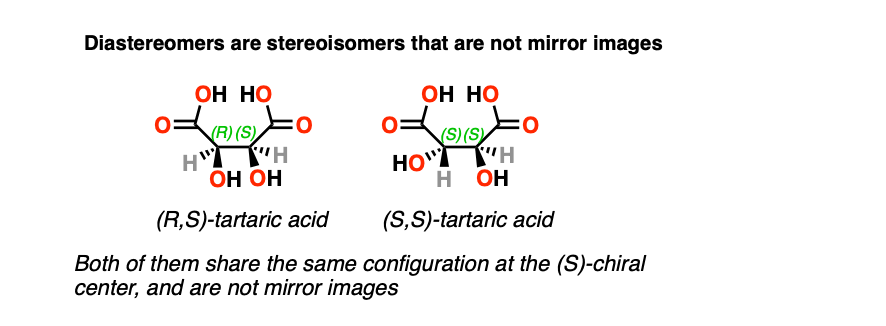
Another situation which may arise is when a molecule has a stereoisomer which is not a mirror image. This commonly occurs when a molecule has more than one chiral center.
Stereoisomers which are not mirror images are said to be diastereomers.
The classic example is (R,R) tartaric acid and (R,S) tartaric acid, which are stereoisomers of each other, but are not mirror images. Note that they each share an (R) configuration at one chiral center. (Enantiomers will always have completely opposite R,S designations)
These two molecules have different physical properties – different melting points, boiling points, solubilities, and in the case of chiral molecules, will completely differ in the extent to which they rotate plane-polarized light.
3. “A Racemic Mixture Is An Equimolar Mixture…” ?
An equimolar mixture for our purposes just means a mixture that consists of an equal number of moles of two components, usually dissolved in a solvent.
A 50:50 ratio or 1:1 ratio of enantiomers is equimolar.
A 60:40 or 20:80 ratio is not.
Recall that enantiomers have identical physical properties – (e.g. melting point, boiling point) except that they rotate plane-polarized light to an equal and opposite extent. [For more discussion: Optical Rotation, Optical Activity, and Specific Rotation]
Therefore, an equimolar mixture of two enantiomers in an achiral solvent will by necessity have an optical rotation of zero, and fulfill our definition of a racemic mixture.
A non equimolar-mixture such as 0.7 mol of (S)-butan-2-ol and 0.3 mol of (R)-butan-2-ol will have an excess of one enantiomer, be optically active, and not be a racemic mixture.
Note that an equimolar mixture of diastereomers is not a racemic mixture!
4. “A Racemic Mixture Is Optically Inactive” ?
A mixture that is Optically Inactive does not rotate plane-polarized light.
Generally, if you have an equimolar mixture of two enantiomers, you will end up with a racemic mixture as a consequence*, since each enantiomer rotates plane-polarized light to an equal and opposite extent. If they are present in equal amounts, there will be no net rotation.
*unless we do something kinky like employ a chiral solvent, or impart some other chiral influence to the solution. Which we won’t do, 99.99% of the time.
5. Racemic Mixtures Through Combining Optically Pure Enantiomers
First, a racemic mixture can be made by adding together an equal number of moles of two enantiomers. If I take 0.5 moles of (R)-butan-2-ol and 0.5 moles of (S)-butan-2-ol, I have made 1.0 moles of a racemic mixture of butan-2-ol.
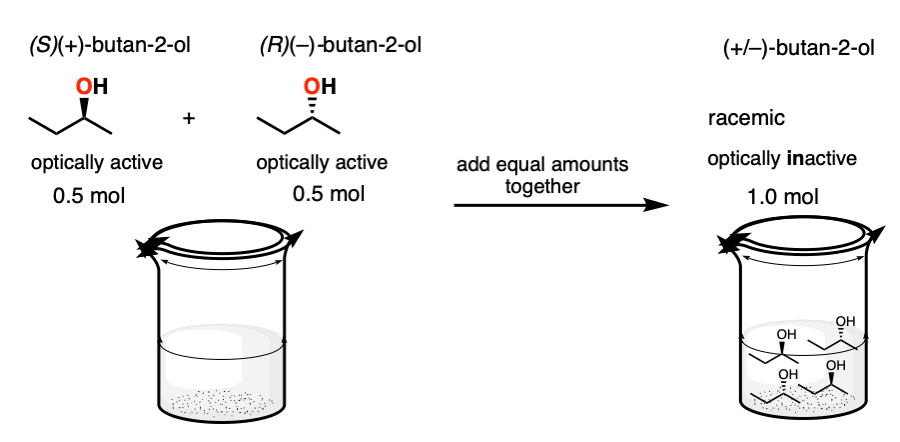
Separating enantiomers is a lot of work. Since time is money, a pure enantiomer will generally be much more valuable than a 1:1 mixture with its enantiomer.
So making a racemic mixture through the addition of equal amounts of enantiomers is generally something done accidentally (e.g. as a consequence of not labeling your vials clearly enough) and accompanied by lots of swearing.
Note – (S)-(+)-butan-2-ol sells for $58.70 / g. (R)-(–)-butan-2-ol sells for $62.10 /g . Racemic butan-2-ol sells for $0.56 / g. So making a racemic mixture is a very efficient method for wealth destruction!
6. How Do You Know If A Reaction Will Make A Racemic Mixture?
More commonly, racemic mixtures can form through reactions which form a new chiral center in the absence of any chiral reagents.
Matt has a nice formulation of this principle, which he calls the “Preservation of Optical Inactivity“.
An optically inactive starting material, reacting with optically inactive reagents will result in an optically inactive product. Optical inactivity is conserved.
Now we come to the big question.
How do you know if a reaction will form a racemic mixture?
I have bad news. Unfortunately, there are no “quick and dirty” rules to follow, other than the (circular) requirement that the product must be an equimolar mixture of enantiomers.
Determining whether a reaction gives you a racemic mixture or not depends on
- The starting material (a.k.a. “the substrate”)
- The pattern of bonds formed/broken for the reaction
You will also need to apply three main skills on a case-by-case basis:
- You have to know what bonds are formed and broken by the reaction, and (especially for alkenes) know its pattern of “regioselectivity” and “stereochemistry”.
- You have to be able to apply this pattern to the starting molecule to determing the correct product(s)
- Assuming that two (or more) products are formed, you have to be able to accurately determine the relationship between these molecules (enantiomers, diastereomers, or other). If only one product is formed, it can’t be a racemic mixture.
[For more discussion, see: “Enantiomers vs. Diastereomers vs. The Same? Two Methods For Solving Problems“
It should come as no surprise that these types of questions are great fodder for exam questions.
Because there is no “quick and dirty” rule, I think it’s best to give a large number of examples which illustrate the different possibilities.
They are not presented in any particular order, but most of them concern reactions of alkenes, and all of them cover reactions encountered in first semester organic chemistry since this is when these questions are most likely to be asked.
As you go through these examples, here are some key questions to ask:
- What bonds form and break in this reaction?
- Where do the new bonds form? What is the stereochemistry of the new bonds that form? (syn, anti, or a mix of both)
- Is a new chiral center being formed?
- Is there any chiral influence in the starting material or reagent(s) that may result in the product being an unequal mixture of enantiomers?
After going through these examples, we can then try to figure out some general principles.
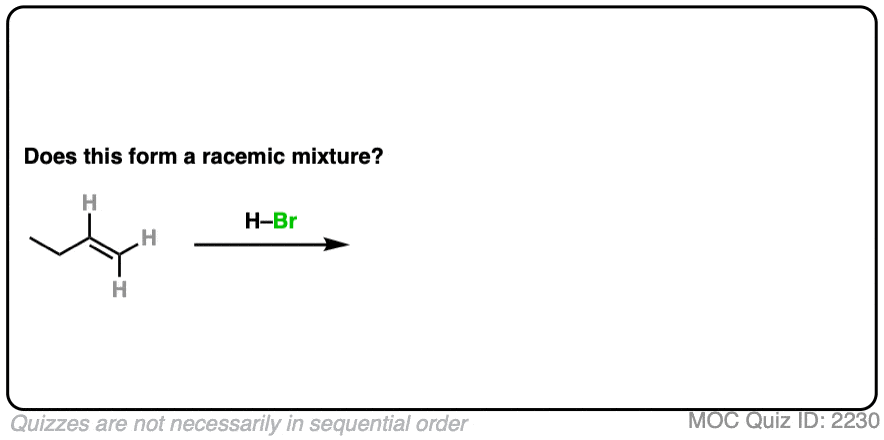
7. Example #1: Alkene Plus HBr
We’ll start with a simple reaction of alkenes: 1-butene plus H-Br.
Does this make a racemic mixture?
 Click to Flip
Click to Flip
This is hydrohalogenation of alkenes, which falls into the “Carbocation Pathway” (Bucket #1) for alkene addition reactions. [See Addition Pattern #1 – The Carbocation Pathway]
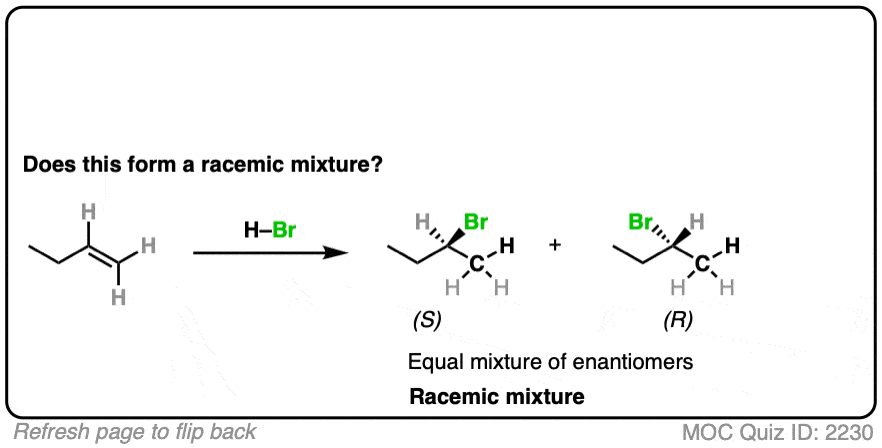
Is a new chiral center formed? Yes. Are there any chiral influences? No.
- The reaction is Markovnikov selective (H goes on less substituted carbon, Br goes on more substituted carbon).
- The reaction will give a mixture of syn plus anti addition.
- Drawing the product, we see that this gives 2-bromobutane, which has a chiral center.
- Since we aren’t using any chiral reagents, we’ll form a 50:50 mixture of enantiomers, which satisfies the requirement for a racemic mixture.
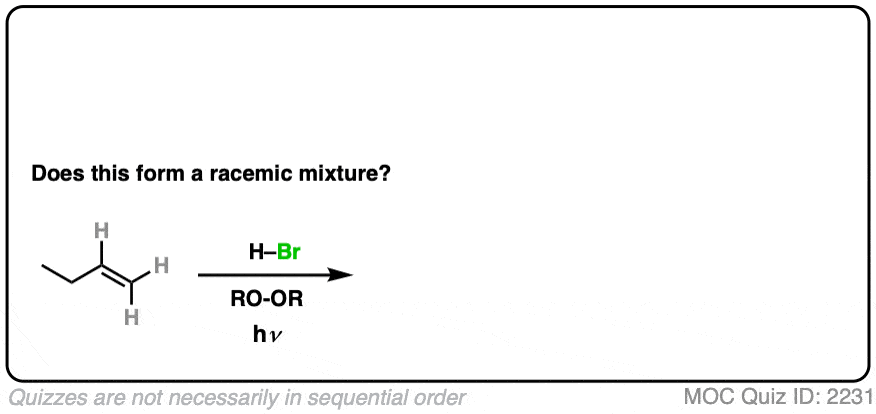
8. Example #2: Alkene Plus HBr (hν)
What about this slight change in conditions, when HBr is added to an alkene in the presence of light (hν).
 Click to Flip
Click to Flip
This is free-radical addition of HBr to alkenes. [See Radical Addition of HBr To Alkenes]
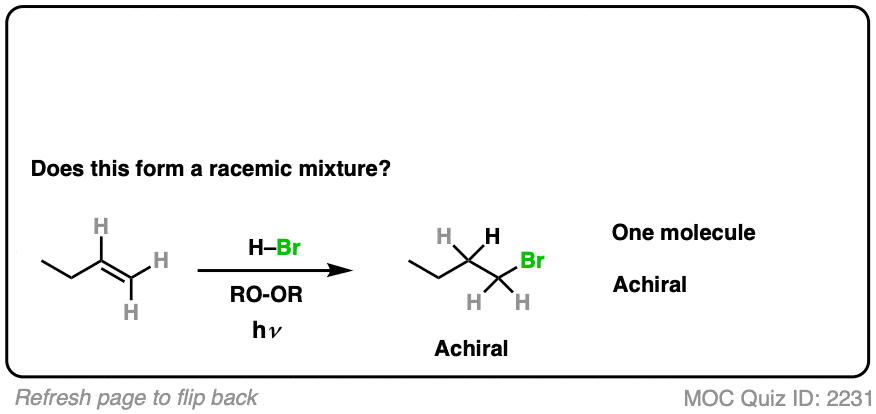
Is a chiral center being formed? No.
- The reaction is anti-Markovnikov selective (H goes on more substituted carbon, Br on less substituted)
- The product is 1-bromobutane, which is achiral. Can’t be a racemic mixture.
How might it be different if we make one little tweak to the starting material? What do you think?
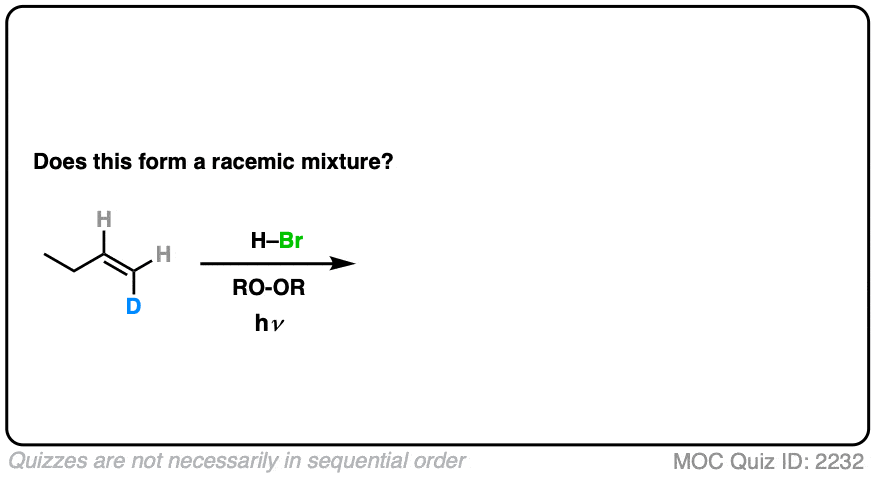 Click to Flip
Click to Flip
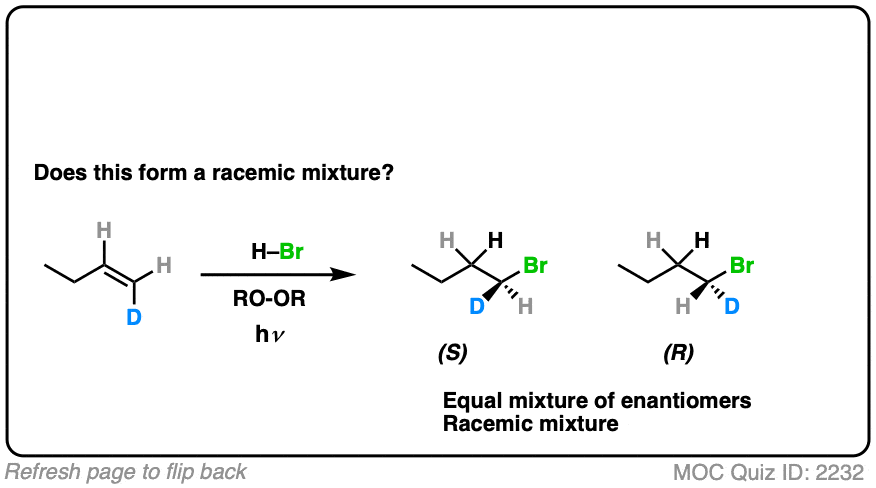
(Note – D is deuterium, the heavy isotope of hydrogen)
9. Example #3: Hydrogenation of Alkenes
Next, let’s look at hydrogenation of alkenes
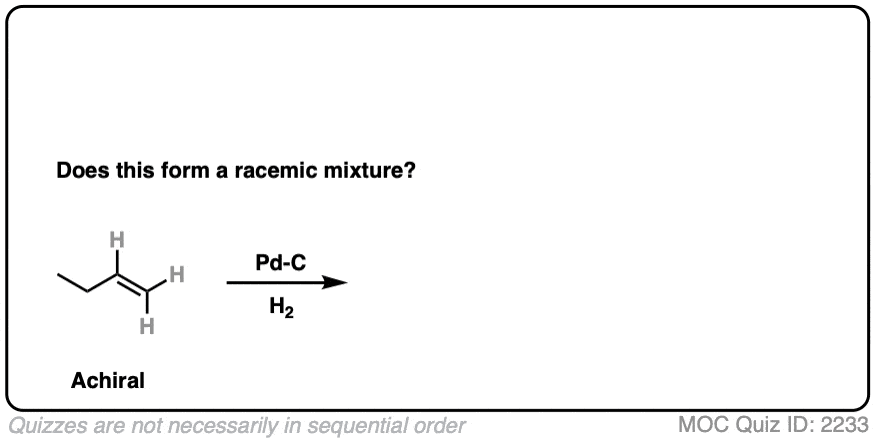 Click to Flip
Click to Flip
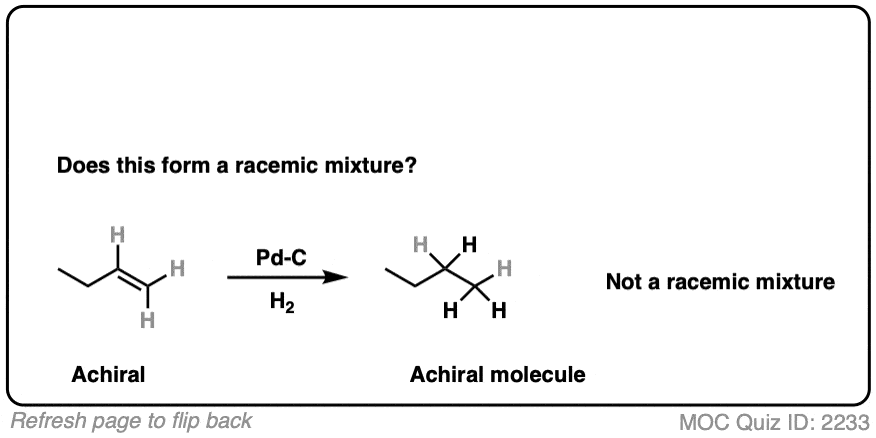
Hydrogenation of alkenes belongs in the “concerted pathway” (Bucket #3 for alkenes). See: [Concerted Alkene Mechanisms – The Concerted Pathway]
Are we forming a chiral center? No.
- Forms C-H bonds on adjacent carbons, oriented syn to each other
- Product is butane, which is achiral. Can’t be a racemic mixture.
What about this one?
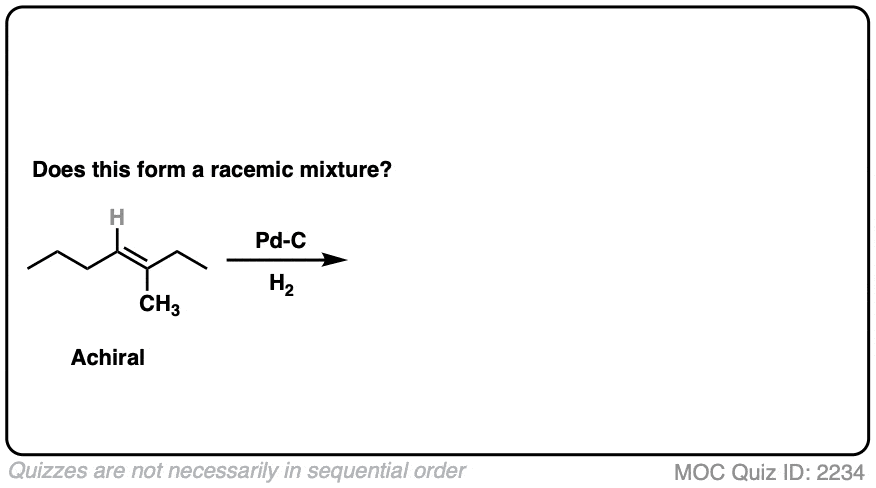 Click to Flip
Click to Flip
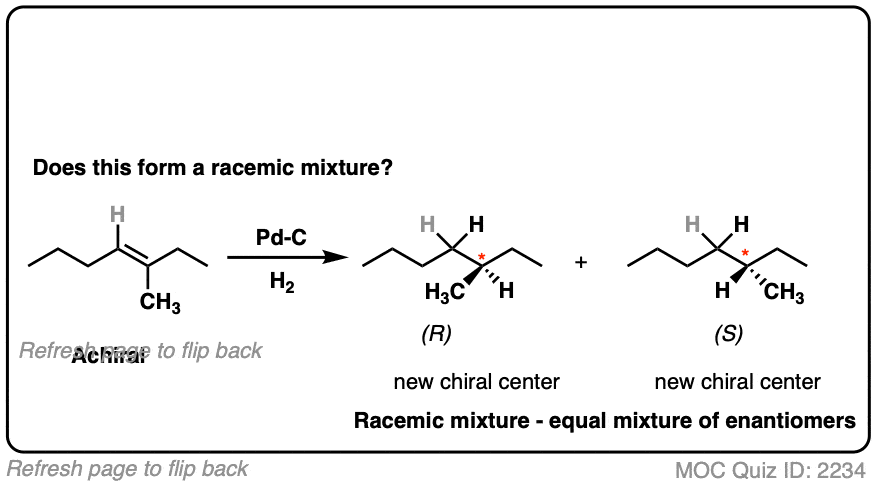
Here we are making a new chiral center. Since there is no chiral influence, the product will be a mixture of enantiomers and therefore be a racemic mixture.
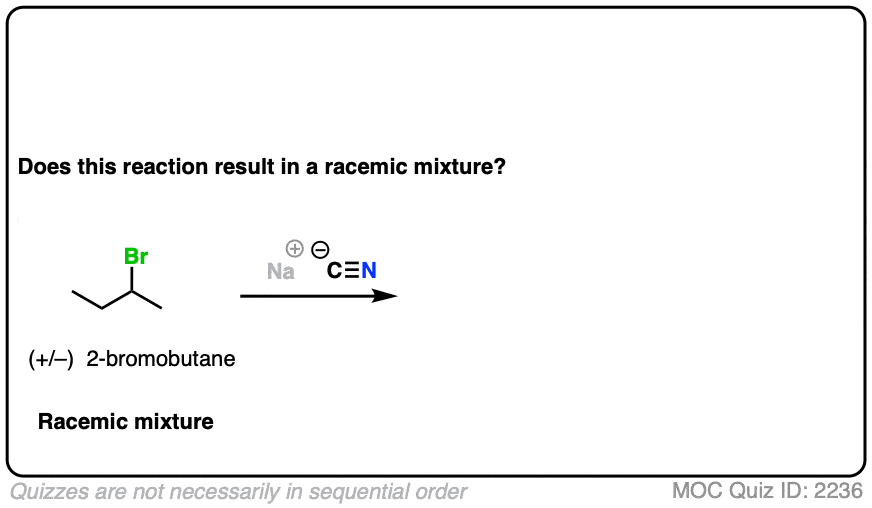
10. Example #4: SN2 of Secondary Alkyl Halides
Let’s look at a substitution reaction next. What about the reaction of (S)-2-bromobutane with NaCN?
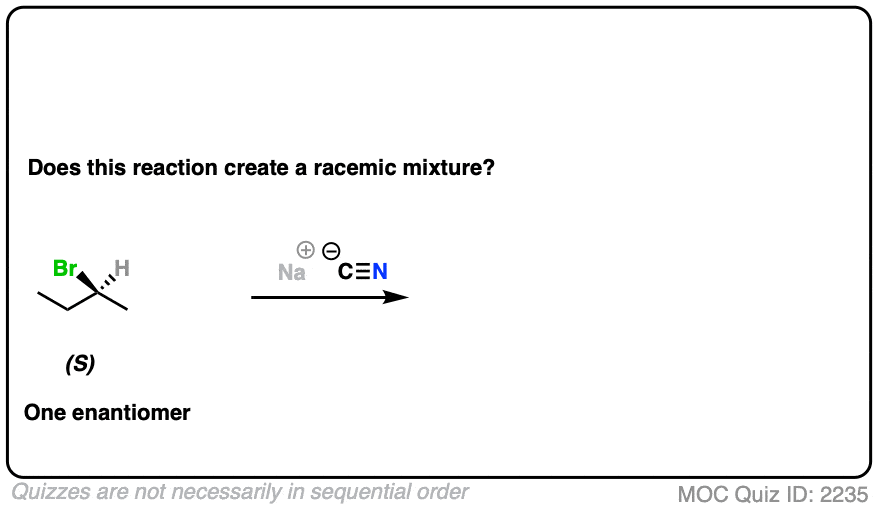 Click to Flip
Click to Flip
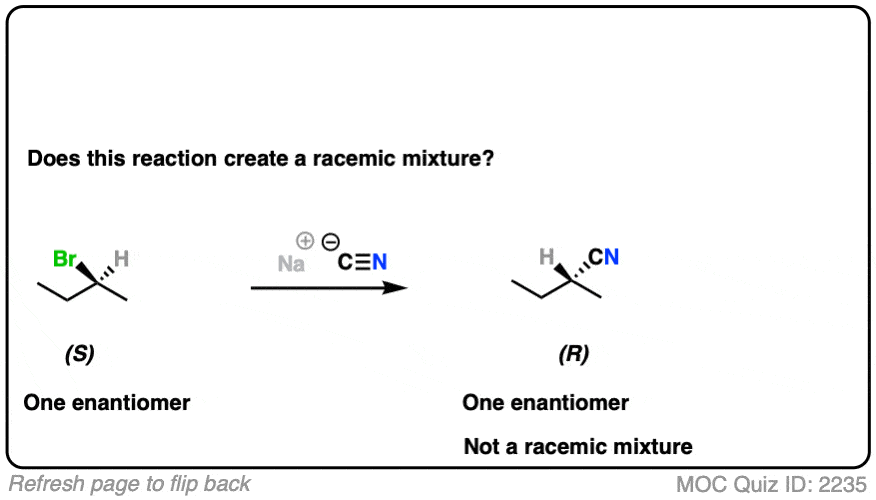
- The reaction is an SN2 reaction, forming C-CN and breaking C-Br
- It occurs with inversion of configuration.
- Our starting material is a single enantiomer and therefore optically active. Since it occurs with 100% inversion, our product will also be a single enantiomer.
- A single enantiomer will be optically active. This therefore can’t be a racemic mixture.
What if we start with a 1:1 mixture of enantiomers instead?
 Click to Flip
Click to Flip
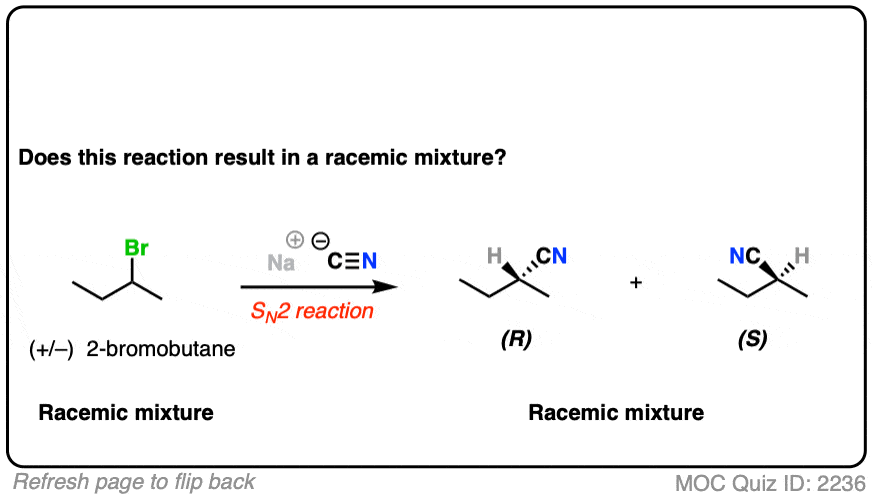
In this case, all the (S)-2-bromobutane will be converted to (R) and all the (R) will be converted to (S)
Our starting material is optically inactive. Our product will therefore be optically inactive.
This is still a racemic mixture.
11. Example #5: SN1 Reaction
How about this substitution reaction of a tertiary alkyl halide?
(Recall that (+/-) denotes the presence of a racemic mixture).
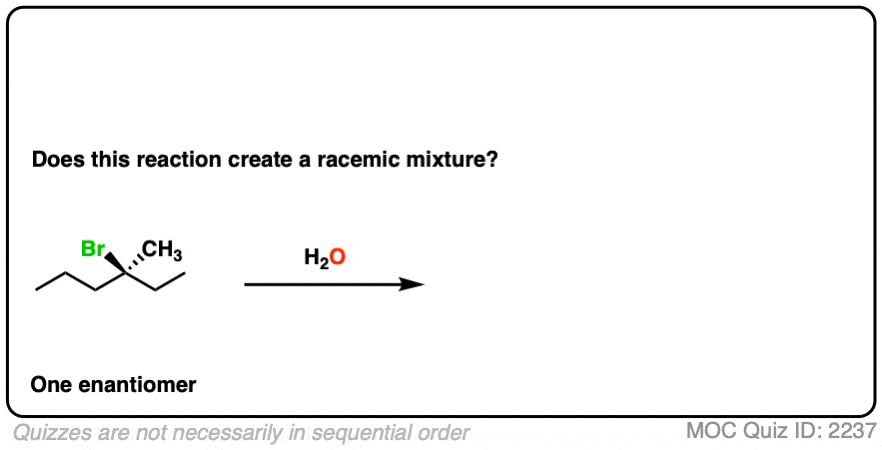 Click to Flip
Click to Flip
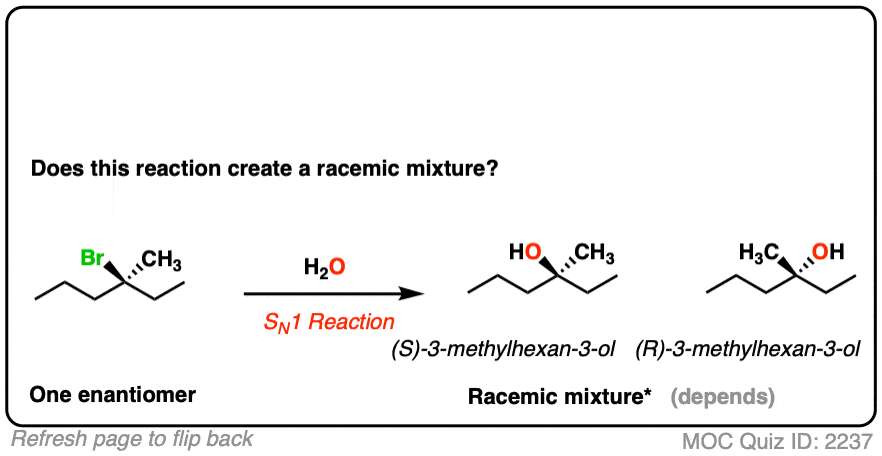
- This is an SN1 reaction, which passes through a planar (carbocation) intermediate.
- Attack on the carbocation can occur via either face, leading to two products [See: Mechanism of the SN1 Reaction]
- Given enough time, this reaction will lead to a racemic mixture [Note 2]
Note that even though the starting material is optically active, we can still end up with a racemic mixture since the product is formed via an achiral intermediate (the carbocation).
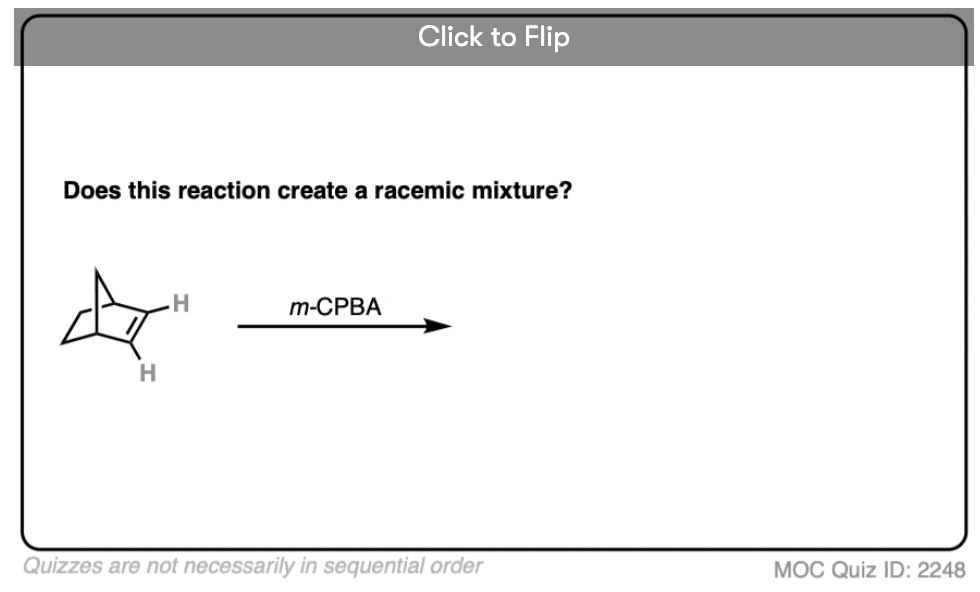
12. Example #6: Pre-Existing Stereochemistry
Sometimes you might already have a stereocenter in the molecule. What happens here?
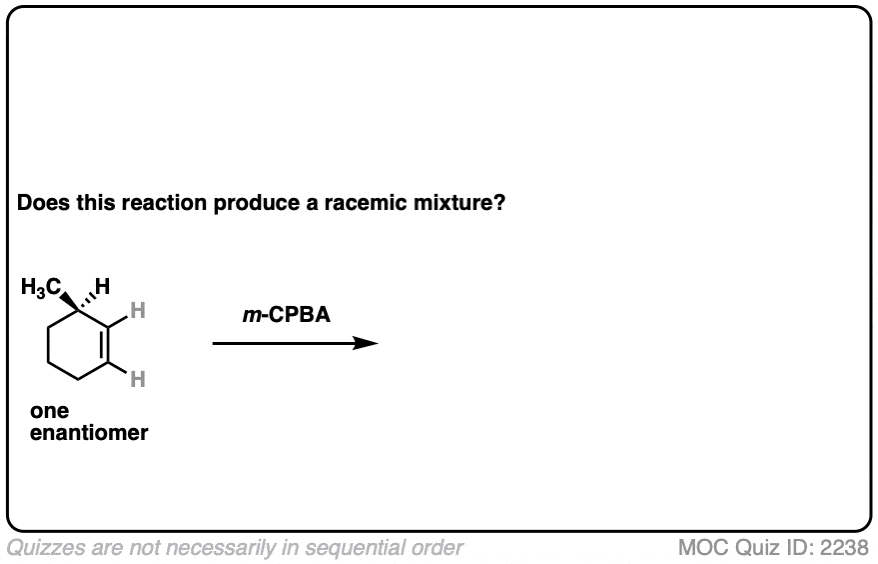 Click to Flip
Click to Flip
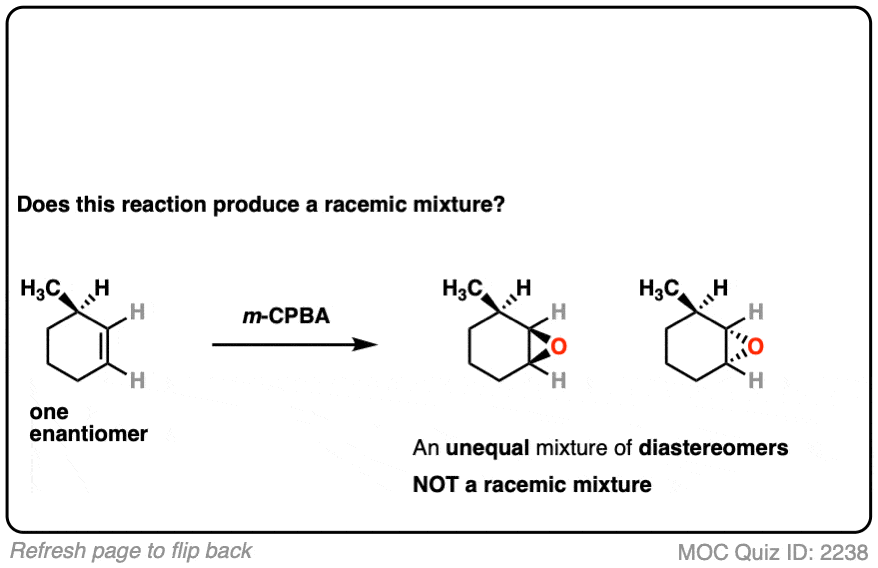
The reaction is epoxidation of an alkene using m-CPBA (m-chloroperoxybenzoic acid). Epoxidation can occur from either face of the alkene.
Note that in this case, however, making a mixture of enantiomers is impossible. That’s because the reaction only affects the alkene, and doesn’t touch the chiral center with the methyl group.
This methyl group will be (R) in both products. So this can’t possibly be a racemic mixture.
[Note: Related question. What if the starting material was itself a racemic mixture?]
13. Examples #7 and #8. Fun with cis-but-2-ene
Does this bromination of cis-but-2-ene produce a racemic mixture?
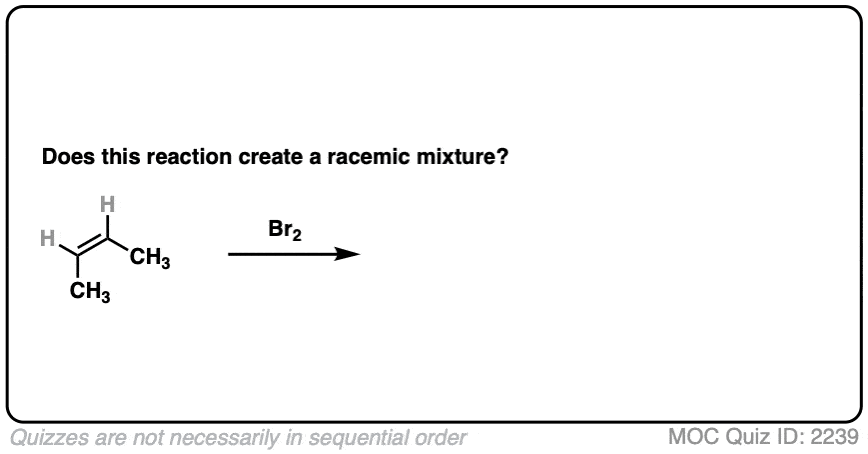 Click to Flip
Click to Flip
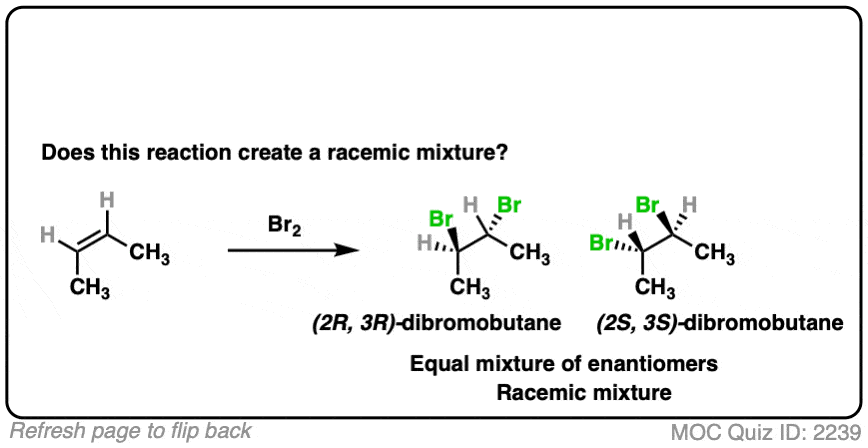
- This is alkene bromination, which proceeds via the 3-membered ring pathway. [See Alkene Addition Pathway #2 – The “Three-Membered Ring” Pathway]
- Two C-Br bonds are formed with an anti relationship to each other.
- In this specific case the products are (2R,3R)-dibromobutane and (2S,3S)-dibromobutane, which makes them enantiomers.
- So this does make a racemic mixture
Same molecule, different reagent. What about dihydroxylation with OsO4?
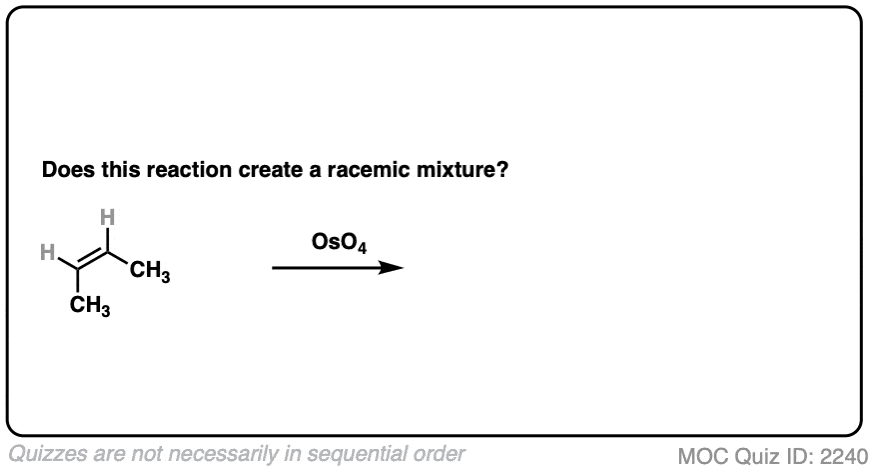 Click to Flip
Click to Flip
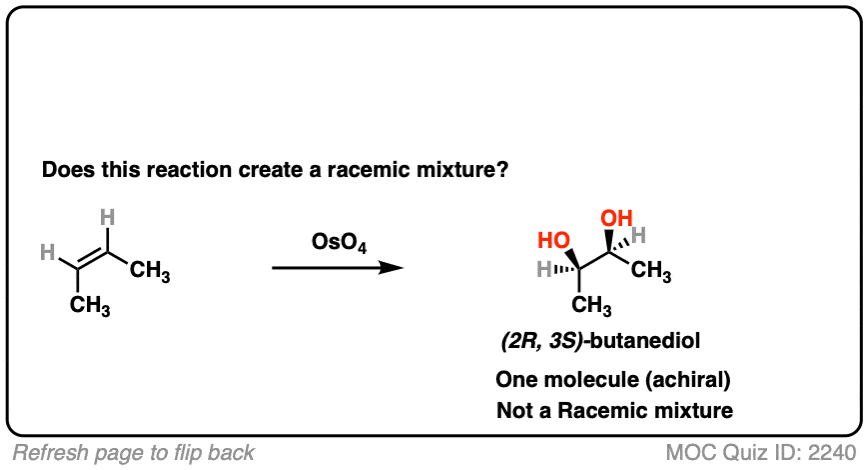
- This is alkene dihydroxylation, which goes through a concerted pathway [See above]
- Two C-OH bonds are formed with a syn relationship to each other
- In this specific case the products are (2R, 3S)butanediol, which is the same as (2S, 3R) butane diol. That is, the product is a meso compound. [See: The Meso Trap]
- The product has two chiral centers, but is itself achiral due to an internal mirror plane. It can’t have enantiomers and is optically inactive.
- This is therefore not a racemic mixture.
Isn’t it interesting that depending on your choice of reagent (Br2 or OsO4) you may obtain either chiral products or a racemic mixture?
14. Examples #9 and #10- Fun with trans-But-2-ene
Slightly different molecule here. What if we perform a bromination with trans-but-2-ene?
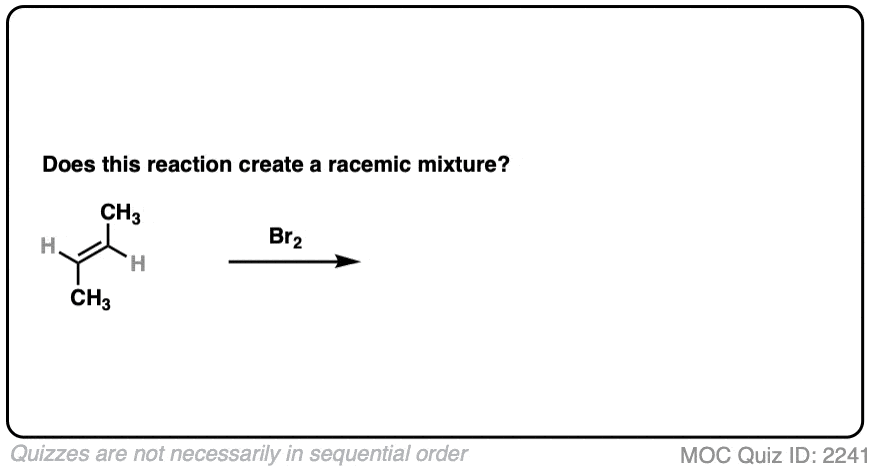 Click to Flip
Click to Flip
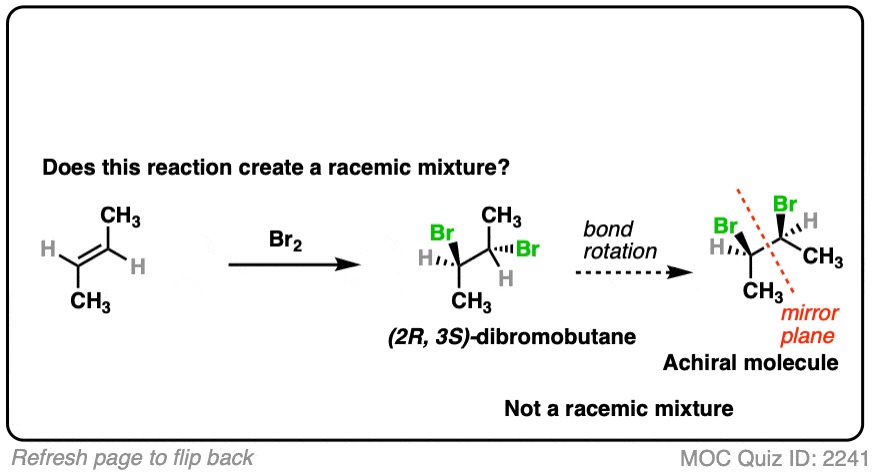
- Bromination gives us two new C-Br bonds in an anti relationship.
- Analyzing the product, we obtain (2R, 3S)-dibromobutane which is the same as (2S, 3R)-dibromobutane due to the internal mirror plane.
- The molecule is achiral. This is not a racemic mixture.
What about dihydroxylation with OsO4?
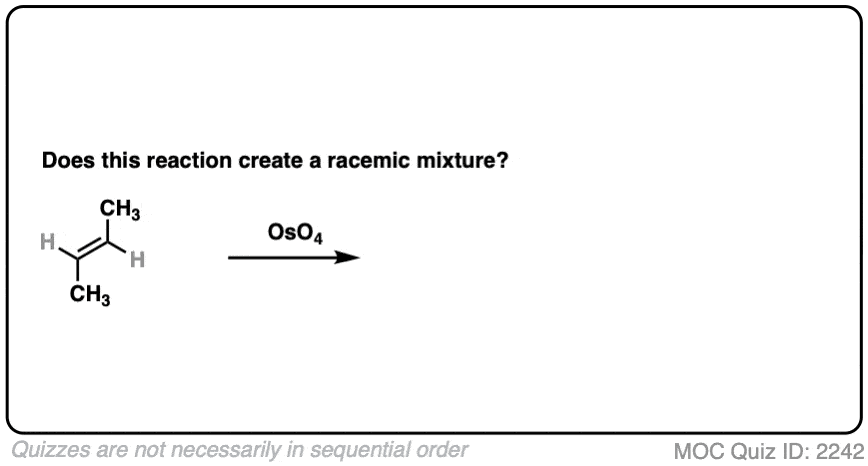 Click to Flip
Click to Flip
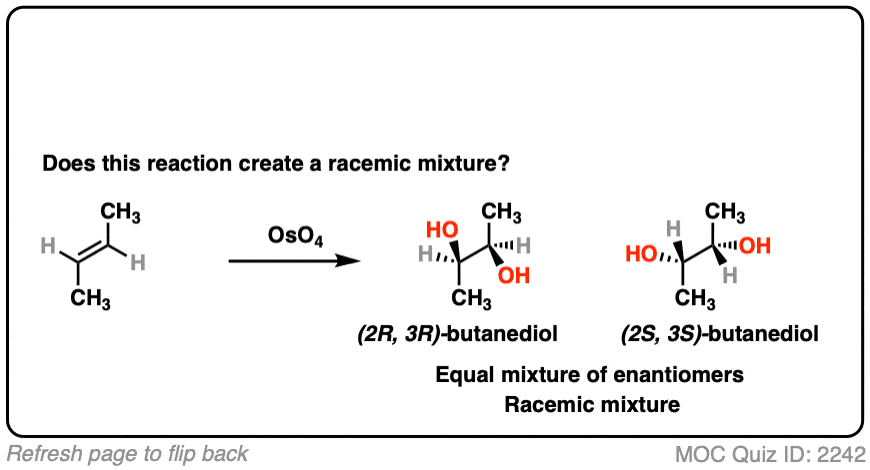
- Dihydroxylation forms two new C-OH bonds syn to each other
- Here we obtain (2R, 3R) butanediol and (2S, 3S) butanediol. They have the exact opposite (R,S) designations and are therefore enantiomers.
- This is a racemic mixture
15. Using A Chiral Reagent
What about this reaction
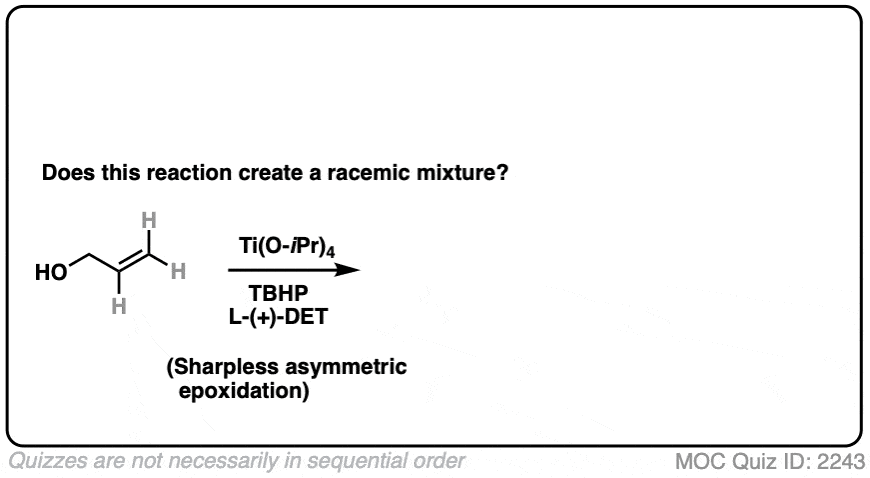 Click to Flip
Click to Flip
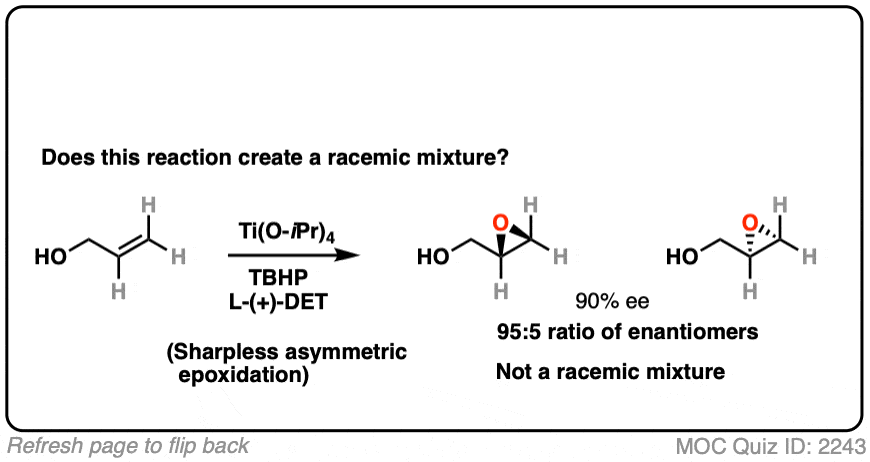
This is a Sharpless asymmetric epoxidation, resulting in a new epoxide. It uses a chiral reagent (a salt of tartaric acid) to make a new epoxide.
The reagent is chiral and in this case predominantly gives one major enantiomer. Since one enantiomer is present in excess, this is therefore not a racemic mixture.
16. Summary
Racemic mixtures are equimolar mixtures of enantiomers that are optically inactive.
They can be made through adding together equal portions of enantiomers or (more commonly) as products of reactions that create a new chiral center in the absence of any chiral influence.
There are no good “shortcuts” to knowing whether a given reaction will form a racemic mixture or not, other than just 1) knowing the pattern of bonds that form and break, and 2) analyzing the resulting products on a case-by-case basis.
If there is a pre-existing chiral center that is not affected by the reaction, then watch out for the possibility of forming diastereomers.
Diastereomers may also form if two (or more) new chiral centers are formed in a reaction.
Notes
Note 1 – On very rare occasions one might use chiral solvents, especially in the context of NMR spectroscopy. Enantiomers in a chiral environment will have slightly different physical properties and will have different chemical shifts.
Note 2 – The SN1 reaction proceeds through an achiral (flat) carbocation intermediate which may be attacked on either side. Some courses teach the SN1 as leading to a “racemic mixture”. In practice whether or not a racemic mixture forms can be dependent on how long the reaction is run for and how reversible it is. If the leaving group is held relatively close to the carbocation in a “solvent cage” then some retention of optical activity may still occur.
Rather than imply that optical activity is lost in all cases of the SN1. Personally I prefer to say it goes with “a mixture of retention and inversion” .
Quiz Yourself!
More quizzes, mostly of weird cases that would have made the article even longer.
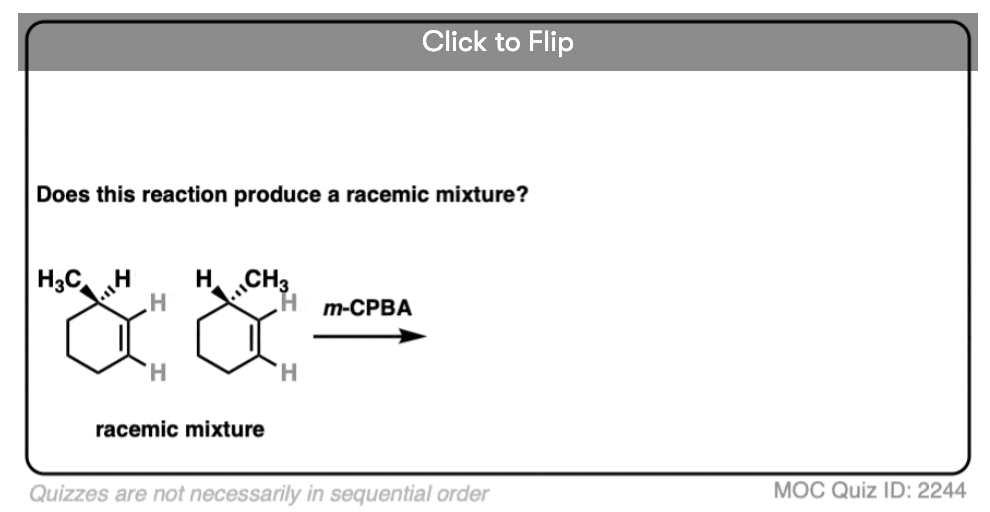
Become a MOC member to see the clickable quiz with answers on the back.
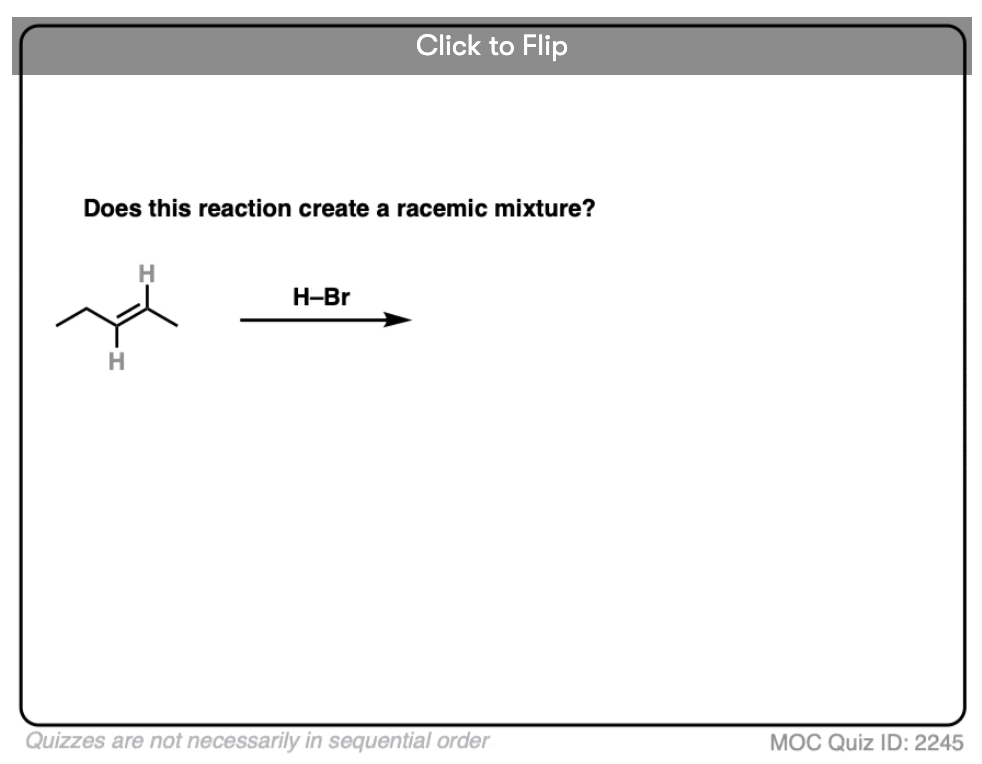
Become a MOC member to see the clickable quiz with answers on the back.
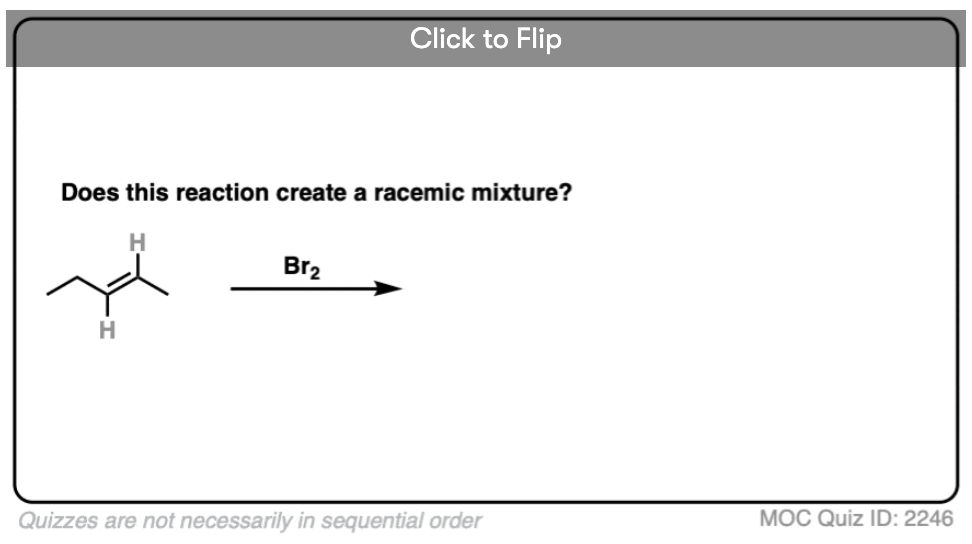
Become a MOC member to see the clickable quiz with answers on the back.
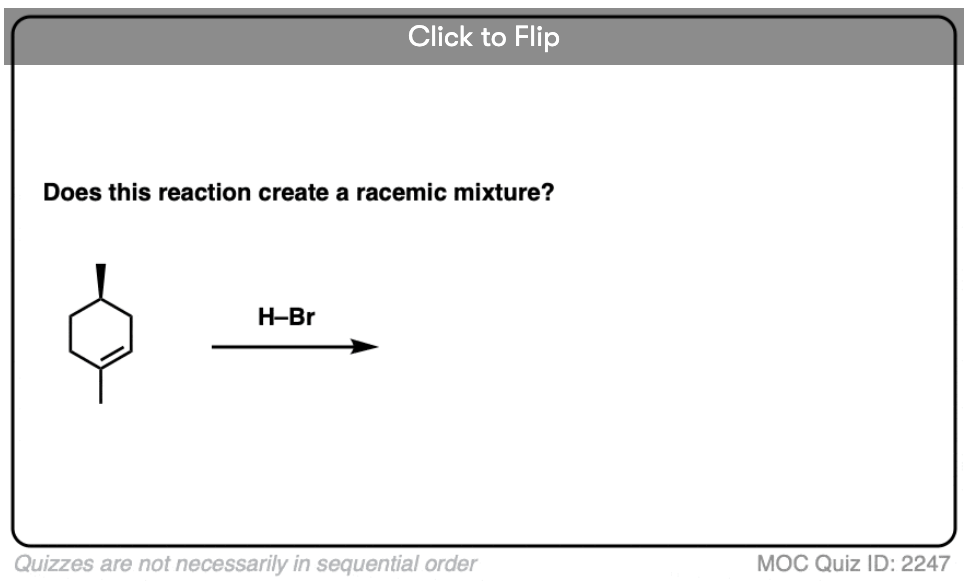
Become a MOC member to see the clickable quiz with answers on the back.
Become a MOC member to see the clickable quiz with answers on the back.
Become a MOC member to see the clickable quiz with answers on the back.
This article has been significantly updated (2022).
Why do these enantiomers form in equal quantities? I mean how this probability works (given enough time)
Hi, it’s just the statistics of very large numbers. When you flip 100 coins, there’s reasonable odds of getting 60:40 heads or tails.
However, one mole has 6.02 x 10^23 molecules. When you flip a coin that many times, the result will almost certainly be 50:50 to several decimal places.
Both enantiomers from recemic mixture has same boiling point and melting point
Great this has taught me. Thanks.
OK, thanks Nabulime.
When I took Masters Organic Chemistry at SUNY Stony Brook “Mirror Image” Molecules were either Racemic or Stereoisomers. Regardless of the name the fact remains…When you Buy a Bottle of 1000mg of Vitamin E which is D,L- Tocopherol Acetate half passes through the Body Unchanged. Useless…BUT if separated the cost of Vitamin E would cost close to the GDP of a small country.
What does it matter if you have racemic mixture of something or not? Does it only matter to a chemist? Because when Primatene Mist disappeared from the world, the only OTC asthma medicine now clearly states “racemic epinephrine” Why? Who cares? Why mention it on the label? Is there a functional difference in drug manufacturing? After all we don’t say “isomer of…” for everything that is a drug and an isomer (the diff between generics is often isomers and it affects people especially in psychiatry, though it’s assumed that it does’t matter). So, why say “racemic” on the asthma med then?
Hi Selma
Great question. Quick answer is: making the racemic version is cheaper and it is almost as effective.
Longer answer below
Epinephrine has a chiral center and thus could exist as one or another optical isomer, or “enantiomer”. When it comes to drugs, enantiomers often have very different medicinal properties. The classic example is thalidomide [where one enantiomer cured morning sickness and the other caused birth defects] , but this is true even of common drugs on the market today such as the antidepressant Celexa (citalopram) where one enantiomer is more active than the other.
The differing effectiveness of enantiomers exists because your enzymes and proteins, for example, also exist as single enantiomers and just like a left hand fits well in a left-handed glove but not a right-hand one, each enantiomer will have a different “fit” within the active site of the protein, causing different effects.
Like any other product, manufacturers of drugs have to be mindful of costs, especially in the case of a non patent-protected medicine like epinephrine.
While it is certainly possible to make epinephrine in either the pure “D” or “L” forms, it adds extra costs to the process. Making it as the “racemic” version (DL) is generally cheaper. For most purposes it is just as effective.
In the case of epinephrine, Wikipedia tells me that the L form is the active component and the D form is inactive. So long as the D form is merely inactive and not actually harmful, it’s cheaper to just make the racemic version and just use double the dose of what you would use for the pure L form.
The only thing to worry about is cases where the other enantiomer is actually toxic. Thalidomide is the classic example. In the case of epinephrine it is not a problem.
I hope this helps
James
So great! I wish I’d found this site sooner!
Glad you find the site helpful Sabrina! Thanks for stopping by!
I have one last question.
Isopulegone is isolated from the reaction and then treated with sodium hydroxide in ethanol.Under the equilibrium conditions of this base-catalyzed isomerization, pulegone is the favored product and, after workup, is isolated as a crude oil (why is pulegone, rather than isopulegone, strongly favored in this equilibrium?
Can someone please answer this question for me? if the starting compound is racemic, will the product also be racemic?)
Yes, with two caveats: 1) your reagent is achiral, and 2) the reaction does not lead to destruction of any stereoisomers. As a counter-example of the latter, oxidation of racemic 2-butanol will give 2-butanone, which is an achiral molecule (not racemic)
Thank you so much
I have one last question.
Isopulegone is isolated from the reaction and then treated with sodium hydroxide in ethanol.Under the equilibrium conditions of this base-catalyzed isomerization, pulegone is the favored product and, after workup, is isolated as a crude oil (why is pulegone, rather than isopulegone, strongly favored in this equilibrium?
Those gloves look so creepy it gave me a panic attack
Thank. The explanation is crystal clear
Thank you for breaking it down with the use of gloves, great technique, I definitely understand what a Racemic mixture is now.
Can we always assume that ketones are always achiral & aldehydes are chiral?
Thanks for that! Somehow seeing it that way makes so much more sense!
I love you and your website. Saved so much headache!!
This is the best explanation I’ve ever read on this topic! Awesome!
Can we predict when we will get a racemic mixture?
this is just what I wanted for my class presentation..thanks a lot
Come be my chemistry teacher please ! love the use of the gloves ! thank you so much
thank you soooooo much >> its is very creative way fo explaining :)
Really creative way of explaining…hatsoff
Thank you so much this makes so much sense!!!
This is exactly what i was thought by proff Anam. All thanks ti this website it has enlighten me the more. All thanks to all who had successfully made this page