Alkene Reactions
Alkene Reactions: Ozonolysis
Last updated: February 12th, 2025 |
Ozonolysis of Alkenes and Alkynes
- Alkenes can undergo oxidative cleavage with ozone (O3) to give carbonyl compounds, cleaving the C=C bond
- The reaction generates an ozonide intermediate, which is then treated with a reducing agent (e.g. dimethyl sulfide or zinc) gives aldehydes or ketones depending on the structure of the starting alkene.
- Less commonly, the ozonide can be treated with an oxidizing agent such as hydrogen peroxide H2O2 (“oxidative workup”) which will convert any aldehydes to carboxylic acids
- Cyclic alkenes are converted into linear products; molecules with multiple alkenes are converted into a mixture of fragments
- More electron-rich (i.e. more substituted) alkenes tend to react faster
- Alkynes can also undergo oxidative cleavage with O3, giving carboxylic acids. Alkynes are less reactive than alkenes towards O3.
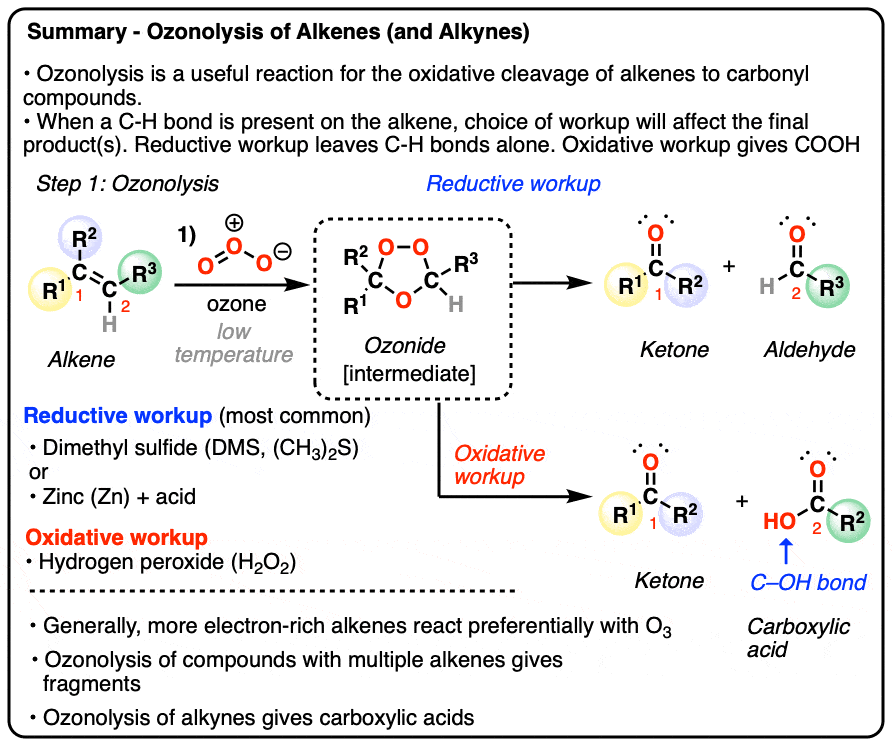
Table Of Contents
- Ozone (O3) Is A Powerful Oxidant For Cleaving Alkenes To Carbonyl Compounds
- Ozonolysis With “Reductive Workup” : All C–H Bonds Are Preserved
- Oxidative Workup of Ozonolysis
- Ozonolysis Of A Cyclic Alkenes Results In A Chain With Two Carbonyls
- Ozonolysis Of A Compound With Multiple Alkenes Results In Fragments
- Mechanism of Ozonolysis
- Oxidative Workup Mechanism
- Summary: Ozonolysis of Alkenes
- Notes
- Quiz Yourself!
- (Advanced) References and Further Reading
1. Ozone (O3) Is A Powerful Oxidant For Cleaving Alkenes To Carbonyl Compounds
Ozone (O3) is a form of molecular oxygen containing three oxygen atoms, in contrast to the the more familiar dioxygen (O2) which we all know and breathe. [O3 and O2 are allotropes of oxygen, just as diamond and graphite are allotropes of carbon]. It has a distinctive sharp, almost metallic odor, detectible by the human nose in trace quantities, that will invoke memories of lightning storms and being near high-voltage power equipment.
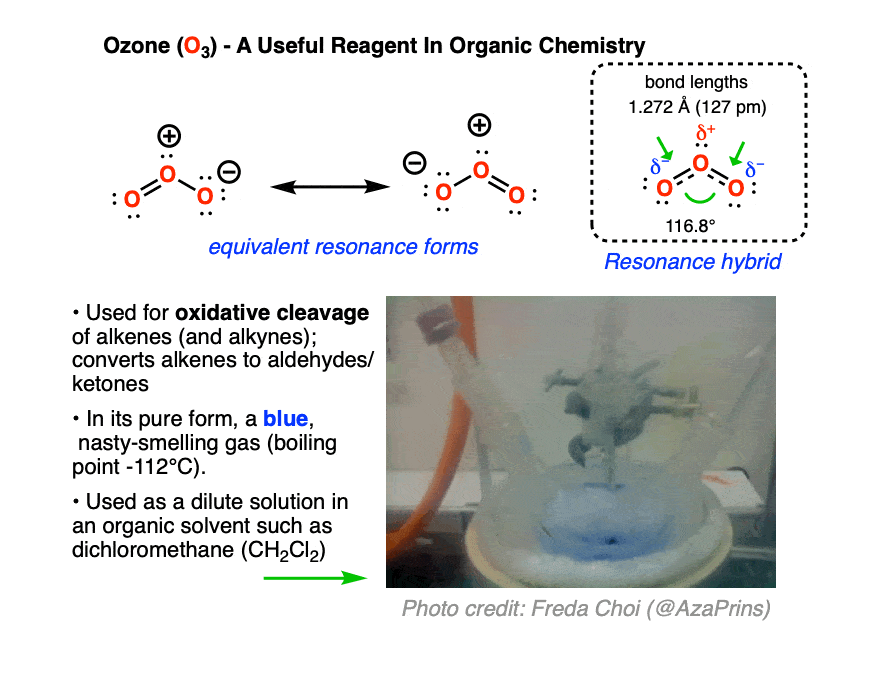
Ozone is a much more aggressive oxidant than molecular oxygen. In organic chemistry, it is useful for oxidative cleavage of alkenes, a reaction that converts alkenes (and alkynes) to carbonyl compounds. It also has many commercial uses, such as disinfecting surfaces, rooms, and drinking water. Ozone can be conveniently generated on demand by passing a stream of O2 through high voltage (8000-15000 V, see this freely accessible article from Org. Syn for the original design) ; household ozone generators are available for purchase on Amazon.
Ozone forms naturally in the upper atmosphere through the interaction of O2 with ultraviolet light, and serves to screen out damaging high-energy ultraviolet rays from hitting the earth’s surface (UV light promotes the pi-pi* transition of an electron from the highest-occupied molecular orbital (HOMO) of ozone to the lowest unoccupied molecular orbital (LUMO) – see UV spectroscopy for more background on how this works).
Less usefully, O3 forms in the lower atmosphere through interaction of oxygen with light, heat, and nitrogen oxides (byproducts of gasoline combustion) and is a component of smog. High levels of O3 lead to rapid degradation of rubber and plastics and also cause respiratory problems.
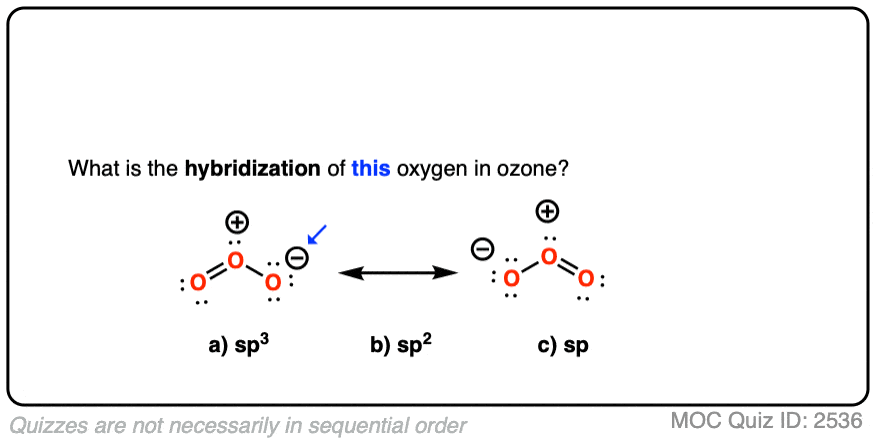
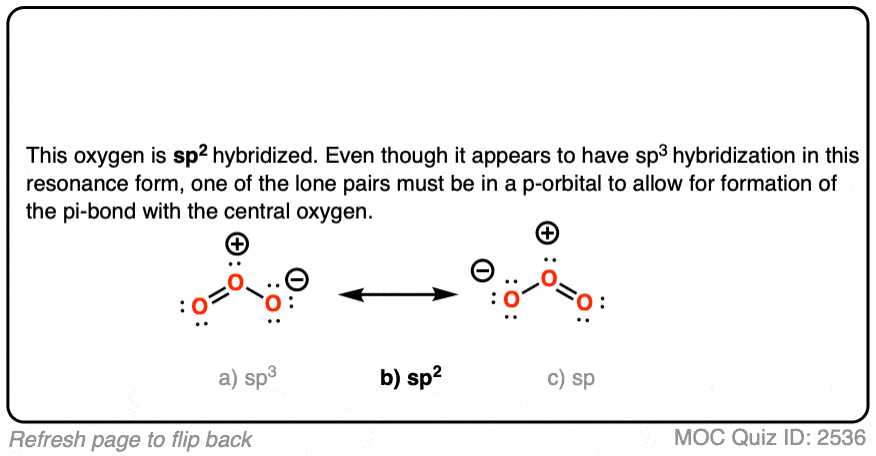
Ozone has two equivalent resonance forms. The resonance hybrid has an O-O bond length of 1.27 Å, intermediate between O=O (1.21 Å) and O-O (1.47 Å) , and an interior bond angle of about 117°, pretty close to the 120° bond angle of an ideal sp2 hybridization. [Note 1]
The hybridization of the terminal oxygen of ozone makes for a good quiz question:
 Click to Flip
Click to Flip
2. Oxidative Cleavage of Alkenes With “Reductive Workup”
When alkenes are treated with ozone, they undergo a reaction known as ozonolysis (ozone, + lysis = breaking), a type of reaction known as oxidative cleavage.
(In organic chemistry, any reaction where a C-H or C-C bond is converted to a C-O bond is classified as an oxidation reaction – see Oxidations and Reductions in Organic Chemistry).
The C=C bond is broken and two new C=O bonds are formed. The resulting C=O groups are known as carbonyl functional groups.
Depending on the structure of the starting alkene, either aldehydes or ketones will be formed. If the alkenyl carbon has two hydrogens attached, formaldehyde (H2C=O) will be formed.
In carrying out an ozonolysis reaction, O3 is added to the alkene at low temperature until the alkene is completely consumed. The beautiful blue color of ozone serves as a convenient indicator – when the blue color persists, you know the reaction is done! [Note 2]
Ozonolysis of an alkene results in an intermediate known as an ozonide (more detail in the “Mechanism” section, below).
Like peroxides, ozonides are potentially explosive (the O-O bond is weak! ) and reasons of chemical safety, they should be broken down to carbonyl compounds through adding additional reagents, a procedure known as the workup. [The explosive nature of ozonides was first encountered in 1873 – Note 3 ]
There are two different families of workups for ozonolysis reactions.
The most common type of workup (“reductive workup“) involves adding a reducing agent to the ozonide, which accepts an oxygen atom and results in the formation of two new C=O groups. Typical reducing agents include dimethyl sulfide [also known as (CH3)2S or DMS), zinc with acid, or triphenylphosphine (PPh3). These safely break the O-O bond of the ozonide and leave all C-H bonds intact.
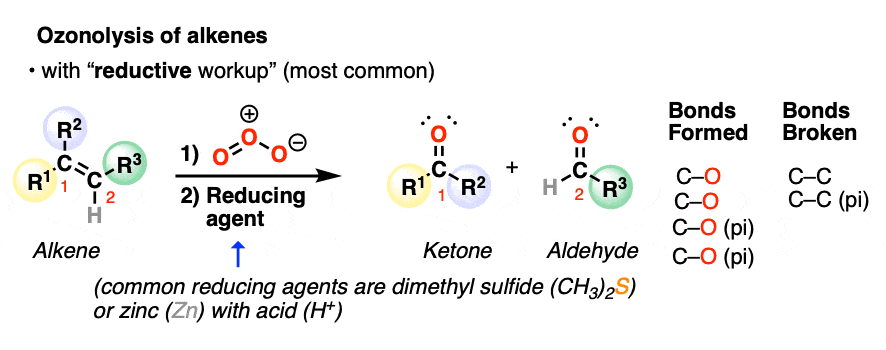
Note the pattern of bonds formed and bonds broken in ozonolysis of alkenes with reductive workup: the C=C bond breaks and two new C=O bonds form.
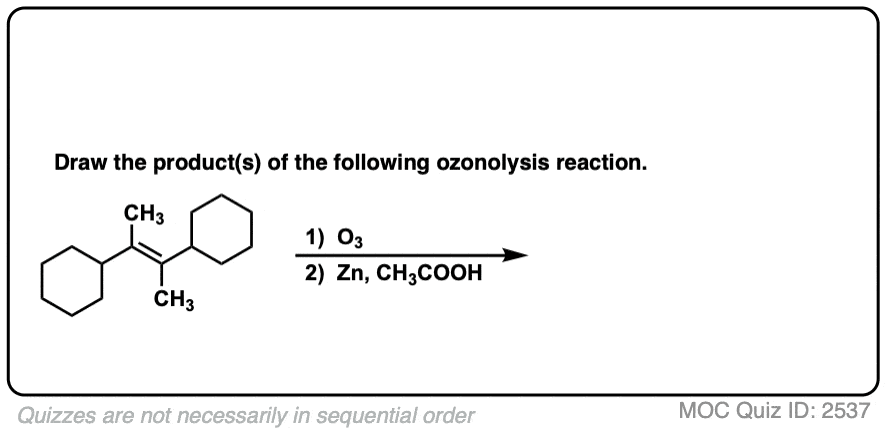 Click to Flip
Click to Flip
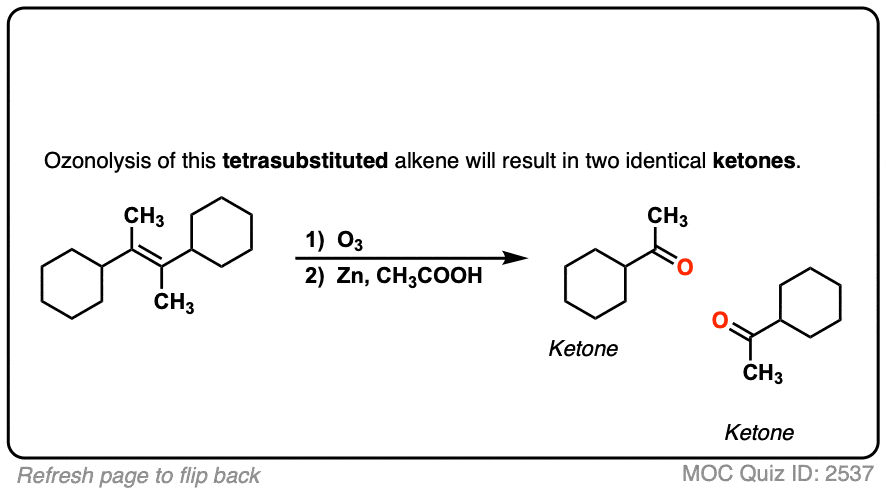
Depending on the structure of the starting alkene, either aldehydes or ketones will be formed. Reductive workup does not alter any additional bonds on the substrate.
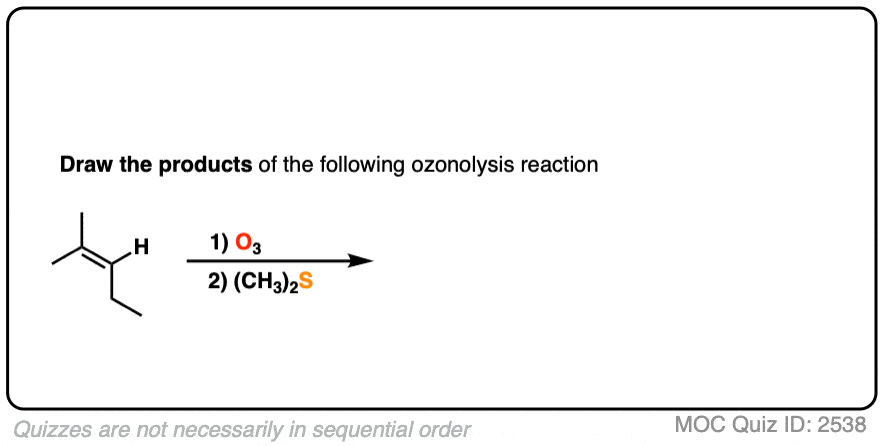 Click to Flip
Click to Flip
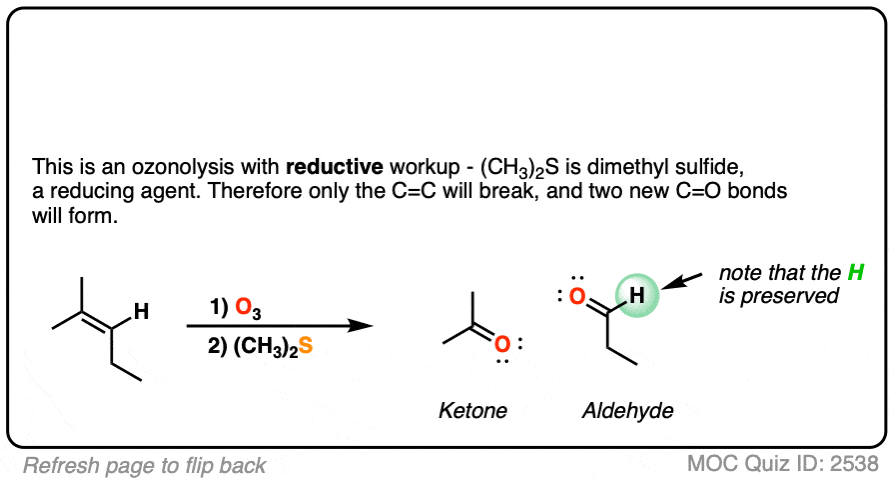
When terminal alkenes (i.e. alkenes that end in =CH2 ) are treated with O3 and subjected to reductive workup, formaldehyde will be formed.
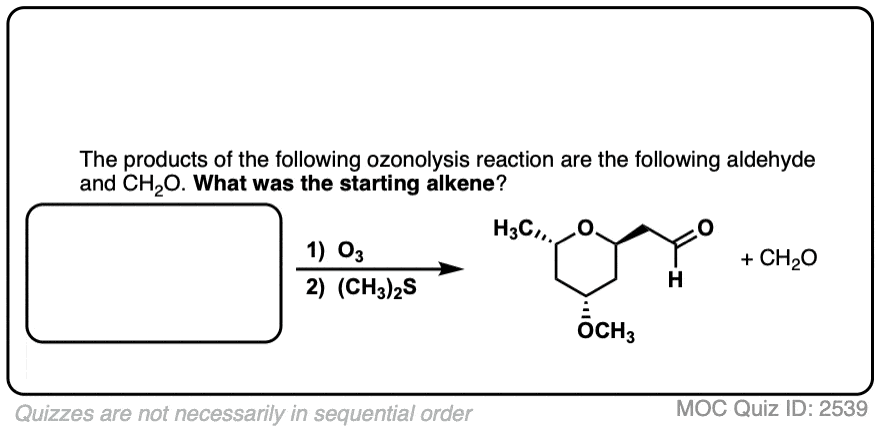 Click to Flip
Click to Flip
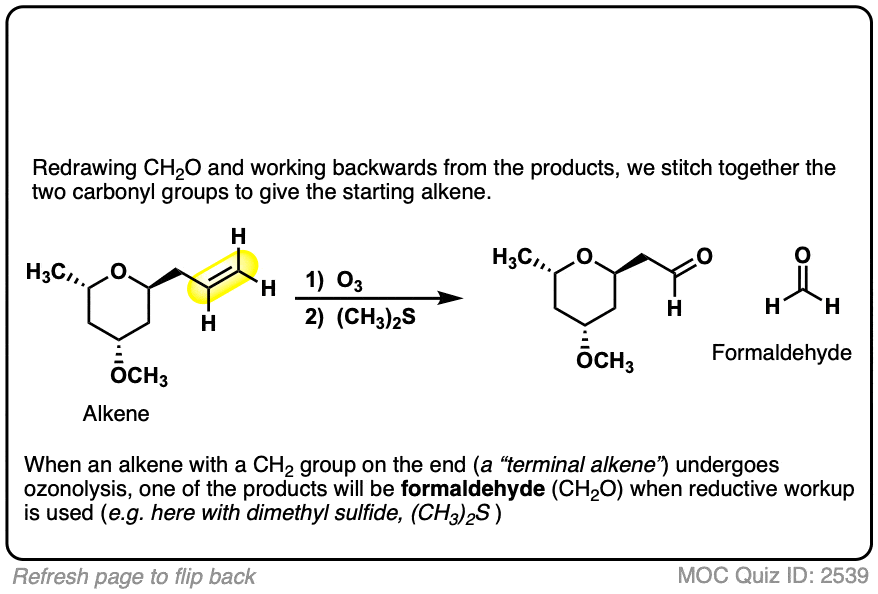
3. Oxidative Workup
A less common workup is known as “oxidative workup”. In this case, an oxidant such as hydrogen peroxide (H2O2) is added to the ozonide. What happens is that any aldehydes that form will be oxidized to give carboxylic acids.
That is, in addition to the C=C bond breaking and two new C=O bonds forming, any C-H bonds on the alkenyl carbons will be converted into C-OH bonds.
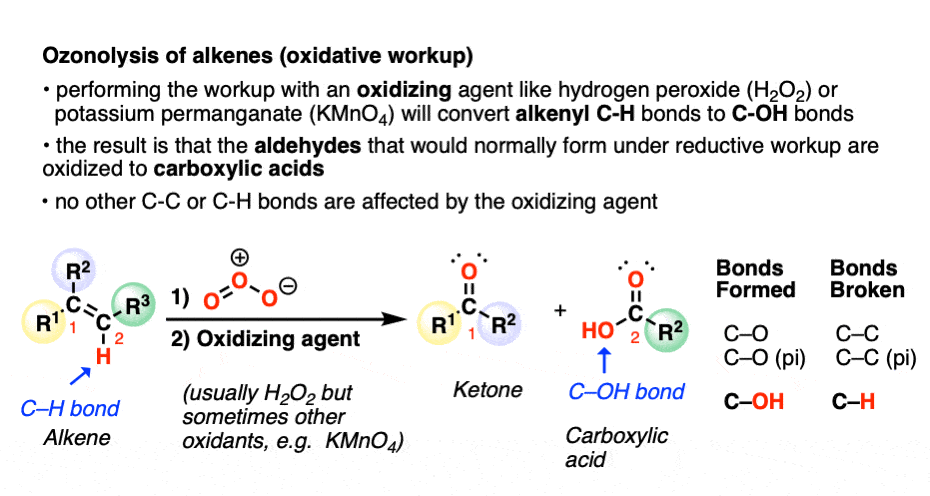
Here is a specific example:
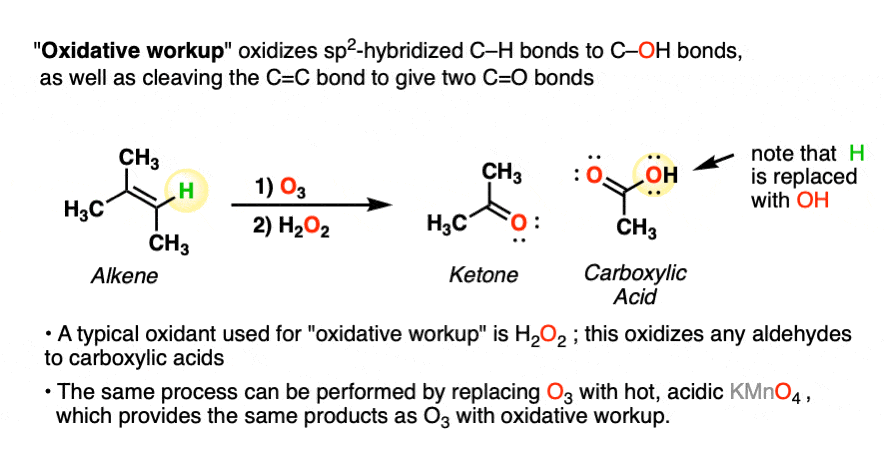
Note that the same transformation can be achieved by treating the alkene with hot, acidic KMnO4.
4. Cyclic Alkenes
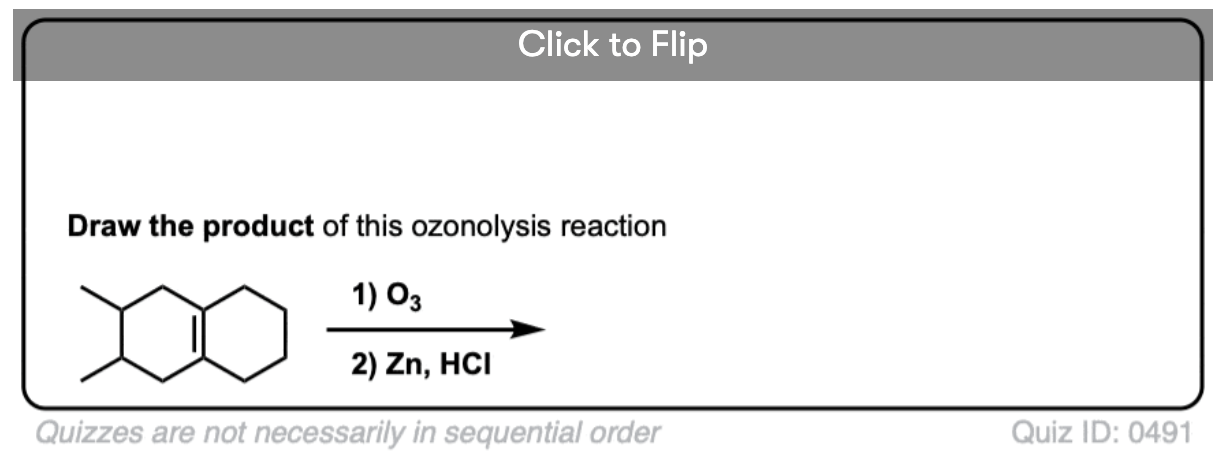
Ozonolysis of cyclic alkenes results in linear chains. For example, this ozonolysis of 1-methylcyclohexene gives the aldehyde-ketone below:
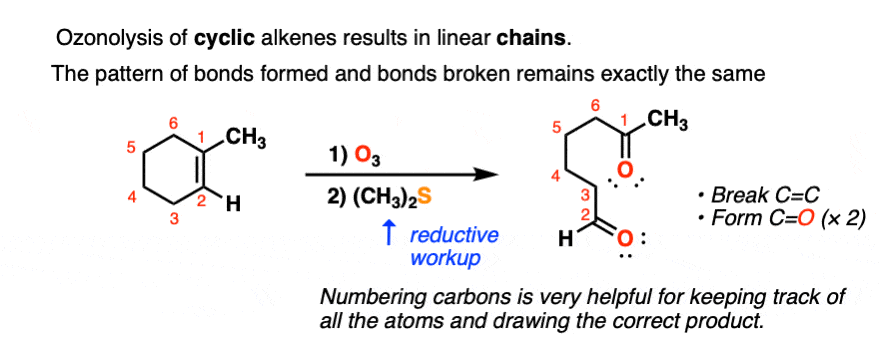
When dealing with these types of examples it can be very helpful to number your carbons and also to take your time with redrawing. My advice is to draw the ugly version first, [See article: Draw the ugly version first] to make sure you get the connectivity right, before trying to re-draw it neatly.
See if you can predict the product of the following ozonolysis.
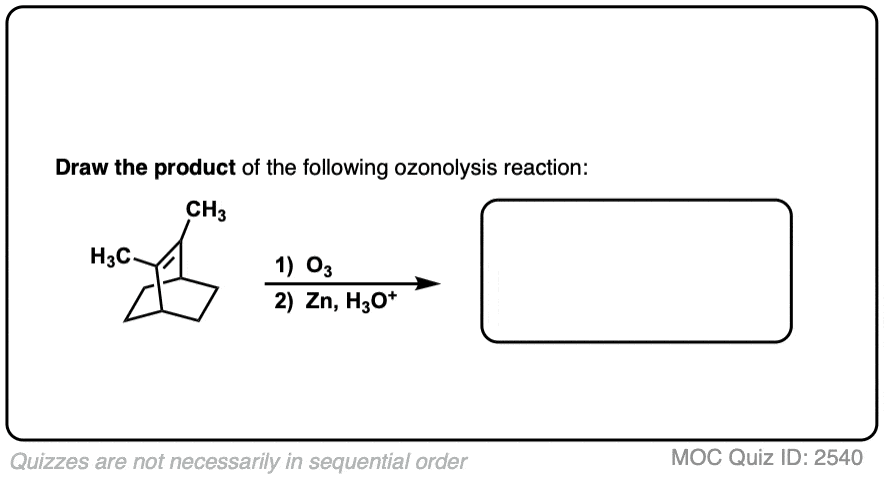 Click to Flip
Click to Flip
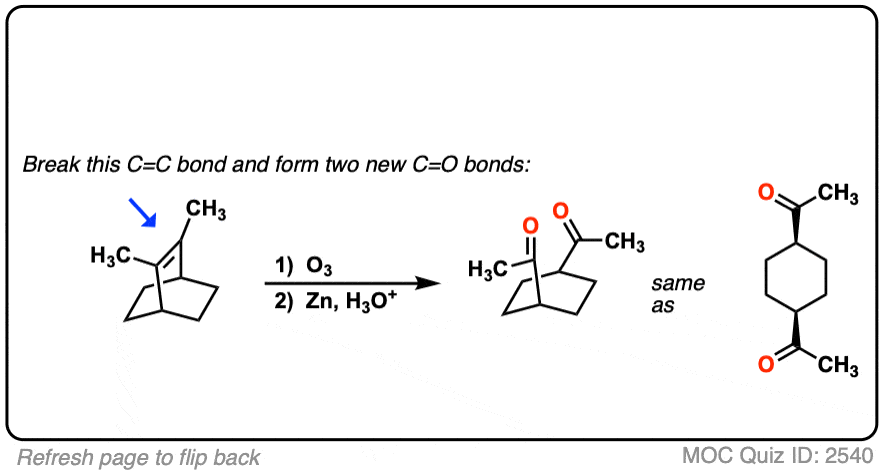
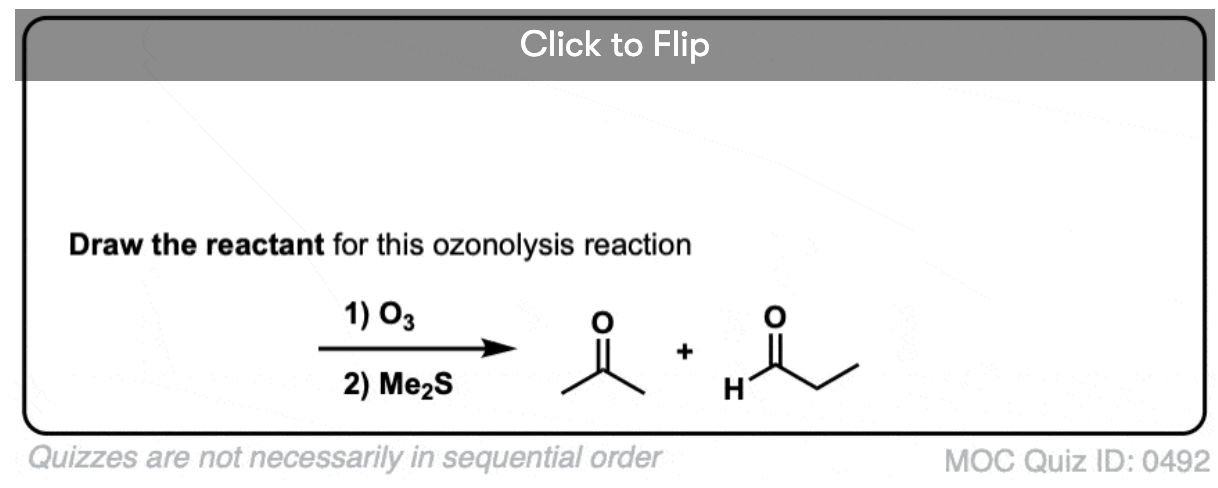
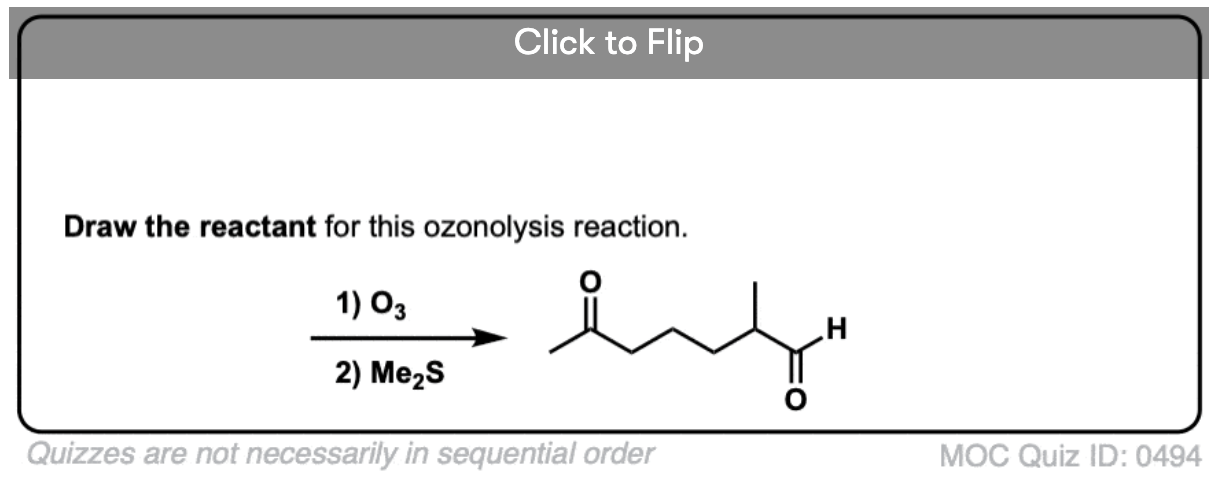
It’s also helpful to be able to think in reverse. Can you work backwards from the following product to the starting alkene?
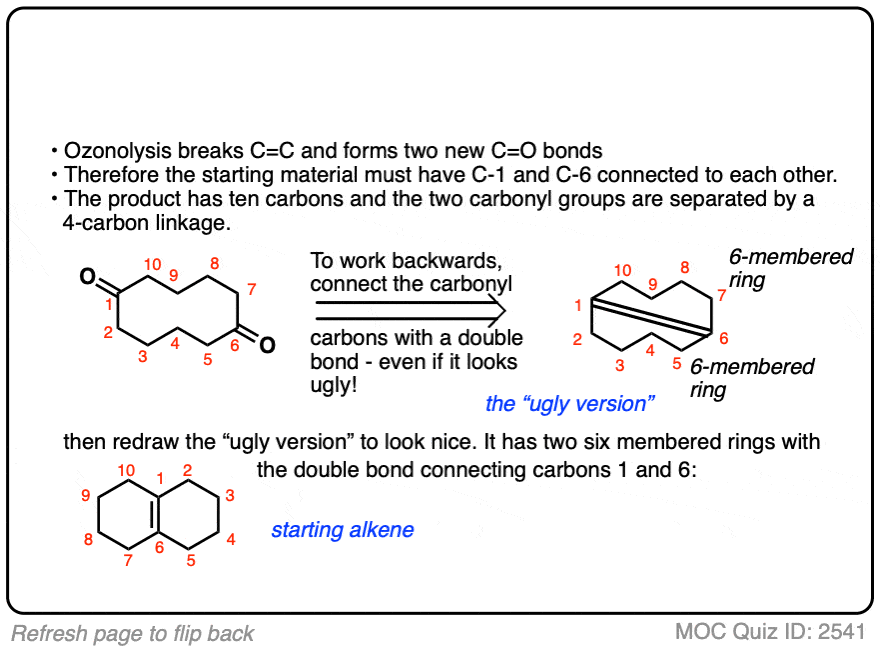
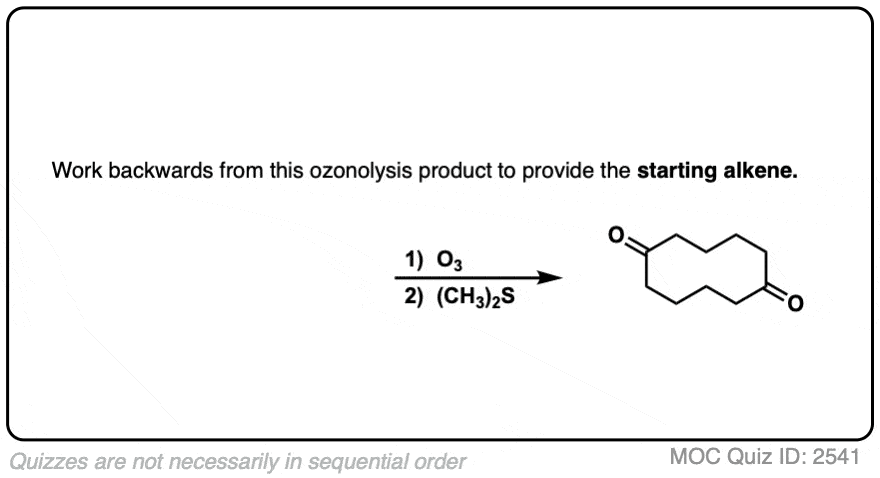 Click to Flip
Click to Flip
5. Ozonolysis of Molecules Containing Multiple Alkenes
When molecules with multiple alkenes are treated with O3, fragments will result. For example treatment of the triene below results in the following collection of carbonyl compounds:
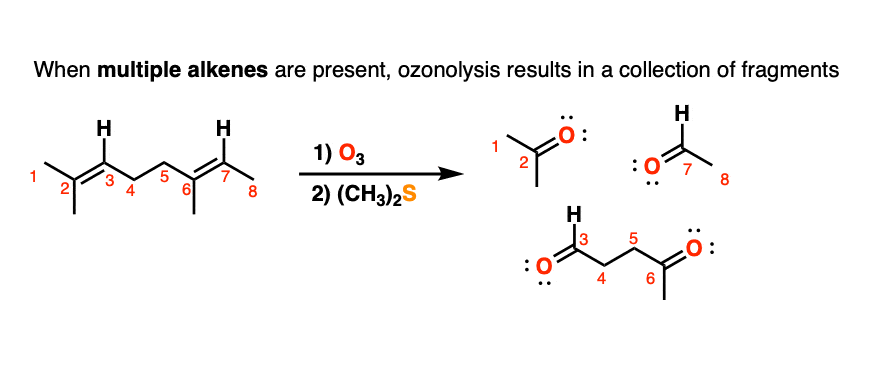
In olden days of yore, before our current powerful spectroscopic methods like nuclear magnetic resonance (NMR), a useful strategy for to determining the structure of unknown compounds was to subject them to cleavage with ozone, isolate and characterize the fragments, and then to do some detective work to put the pieces back together in the proper order. This type of structural analysis is known as degradation. (For a typical example, see Case Study: Structure Determination of Deer Tarsal Gland Pheromone)
An example of a molecule with multiple alkenes that results in fragments after ozonolysis is shown below:
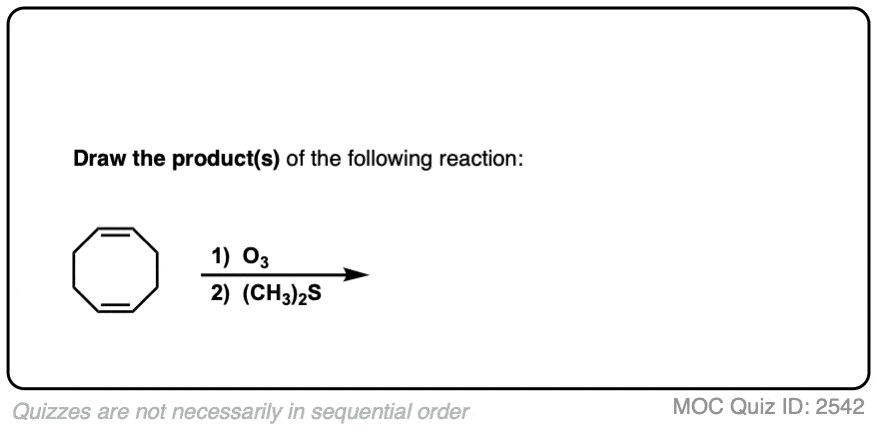 Click to Flip
Click to Flip
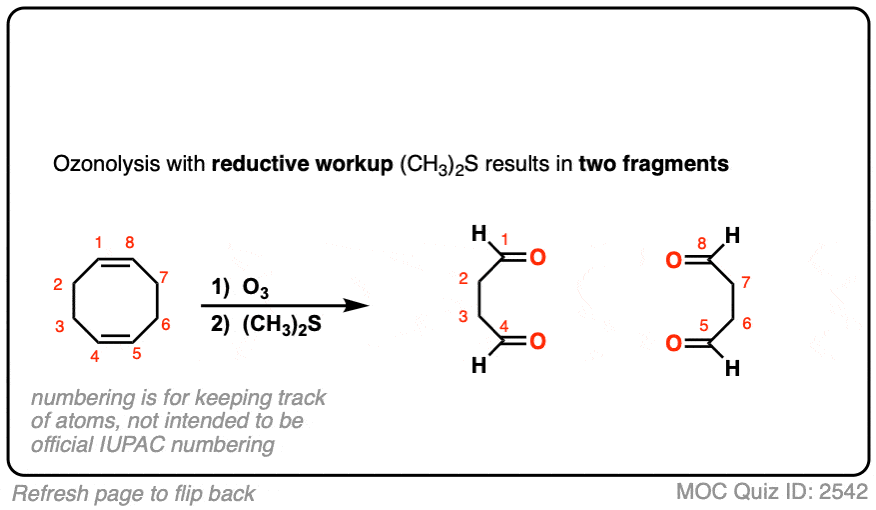
6. Mechanism
The mechanism of ozonolysis took several decades to work out and involves a series of reactions that are not commonly encountered in introductory organic chemistry. So learning the mechanism of ozonolysis is going to feel a lot like memorization without understanding. Sorry in advance.
In the first step, an alkene combines with ozone in a concerted reaction known as a cycloaddition. [The closest thing you will likely encounter to this reaction in introductory organic chemistry is the Diels-Alder reaction, which is a cousin of this process – See post – the Diels-Alder reaction ] [Note 4]
The C-C pi bond breaks, and two new C-O bonds form. An unstable cyclic intermediate known as a “molozonide” forms, which has three consecutive oxygen atoms:
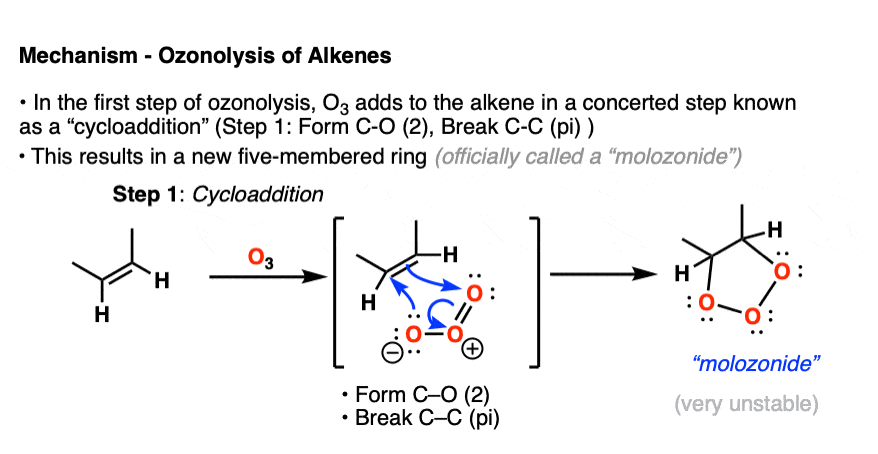
Molozonides have a very short lifetime. In the lab, they can be observed at temperatures around -100°C to -130°C [ref] but quickly break down in a reaction known as a reverse cycloaddition. The central C-C bond breaks, with simultaneous formation of two new C-O (pi) bonds.
Two fragments result. The first one is a conventional carbonyl compound (either an aldehyde or ketone depending on structure). The second one is a zwitterionic structure known as a carbonyl oxide (although don’t worry if you can’t remember the name).
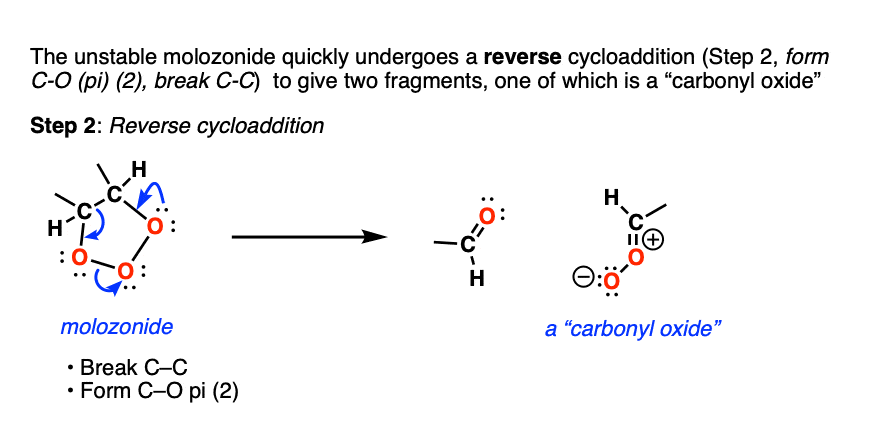
What next? It turns out to be another cycloaddition [Note 5] where the two fragments recombine to give a new five-membered ring containing an ether linkage and a peroxide. This structure is commonly referred to as an ozonide. although sometimes the term “1,2,4-trioxolane” is used.
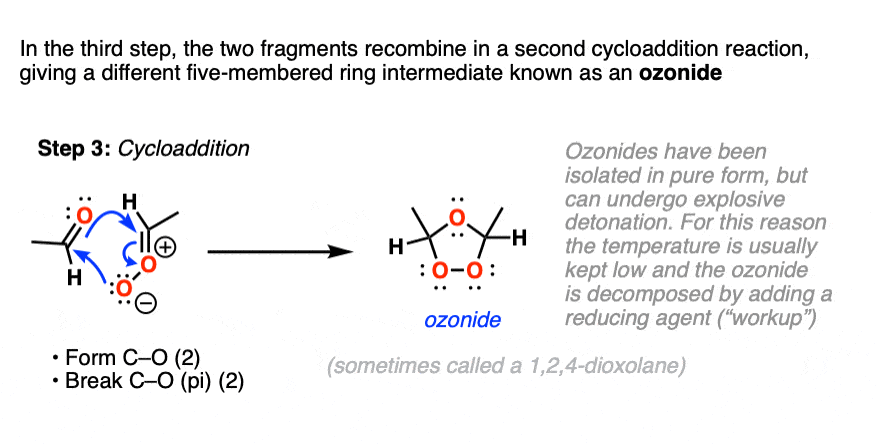
Ozonides can be,
Upon warming, ozonides will break down to give aldehydes/ketones, but because ozonides, just like organic peroxides, can be ” ‘splodey ” as some people would put it, they are best kept cold and in dilute solution, rather than isolated. Apparently they can form nice crystals though!
For this reason ozonides are usually broken down at low temperature by adding a reducing agent, which breaks the weak O–O bond and liberates the two carbonyl compounds, and also destroys any excess ozone.
Two common reagents for reductive workup are dimethyl sulfide (DMS) and zinc.
- Reduction using dimethylsulfide has the advantage of generating benign dimethylsulfoxide (DMSO). To see a plausible mechanism, Hover here for the mechanism or click this link.
- Reduction using acidic zinc results in zinc oxide (ZnO). To see a plausible mechanism, hover here or click this link.
In addition, triphenylphosphine (PPh3) can also be used, but from a practical perspective this tends to be a less popular choice owing to the difficulty of separating triphenylphosphine oxide (PPh3O) from the resulting product.
7. Oxidative workup
Alternatively, allowing the ozonide to warm up in the presence of hydrogen peroxide (H2O2) will lead to the oxidation of any aldehydes to carboxylic acids.
For a possible mechanism (generally not covered in introductory organic) hover here or click this link.
8. Alkynes
Alkynes can also undergo oxidative cleavage with O3 to give carboxylic acids.
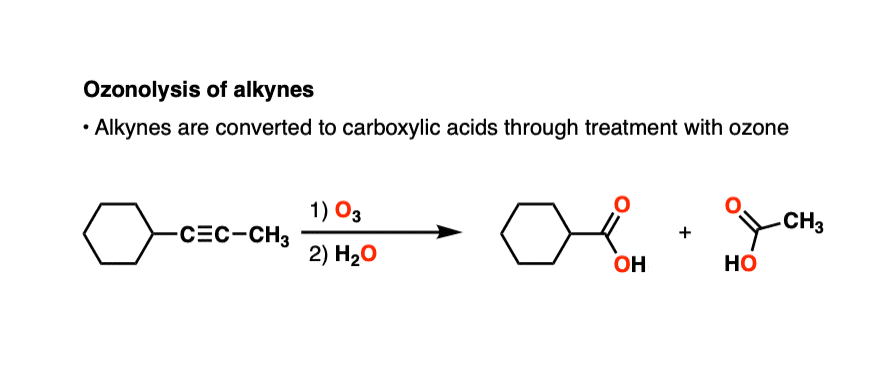
Alkynes tend to be less reactive toward ozone than alkenes. There are plenty of examples of selective ozonolysis of an alkene in the presence of an alkyne. [like here]
Ozonolysis of terminal alkynes (i.e. those that have a C-H bond) results in carbon dioxide. =
Alkynes are more reactive than aromatic rings, however (if you’re in Org 1, the special stability of aromatic rings like benzene is usually covered in Org 2 – see Aromaticity).
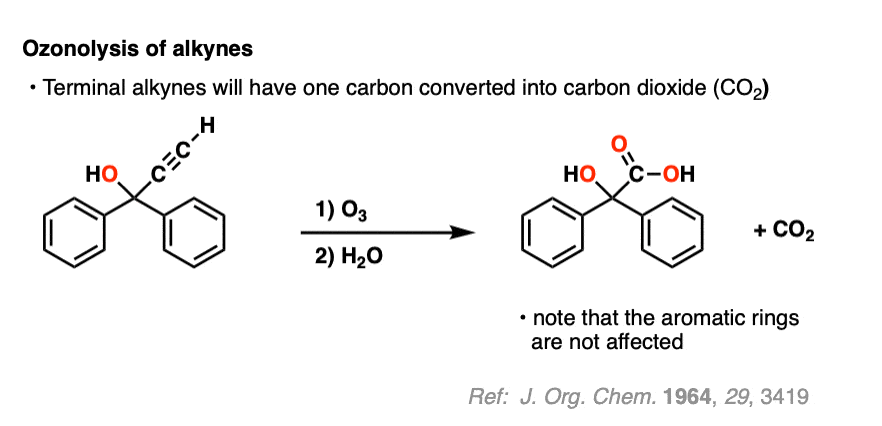
9. Summary
The ozonolysis of alkenes and alkynes belongs to a class of reactions known as oxidative cleavage. Some other reactions in this family include the cleavage of vicinal diols by NaIO4 or Pb(OAc)4, as well as the cleavage of alkenes with hot, acidic potassium permanganate (KMnO4).
- Make sure you understand the difference between reductive workup (leaves C-H bonds alone) and oxidative workup (oxidizes C-H bonds to C-OH bonds)
- Practice examples of the ozonolysis of cyclic alkenes. Don’t forget to count your carbons.
- Practice working backwards from ozonolysis products to the starting alkenes.
- In synthesis problems, be alert to the possibility of using alkenes as precursors to carbonyl compounds.
Notes
Note 1. These values are obtained from microwave spectroscopy. See Hughes, R. J. Chem. Phys. 24, 131–138 (1956). DOI: 10.1063/1.1700813
Note 2. Generally, more electron-rich alkenes (more substituted) undergo ozonolysis more quickly than electron-poor alkenes. When multiple reactive functional groups are present in a molecule, it is possible to obtain good selectivity through the use of appropriate dyes such as Sudan Red (for an example, see here)
Note 3. Apparently the first isolation of an ozonide was made in 1873 by Houzeau, who obtained white, explosive crystals from the oxidative cleavage of benzene with ozone. [Ref]
Note 4. Technically this is a “1,3-dipolar cycloaddition” reaction, a process similar to the Diels-Alder in which a 4 pi-electron component undergoes a cycloaddition with a 2 pi-electron component. One key difference in this case is that it operates with “inverse electron demand”; in contrast to the normal Diels-Alder, which is fastest with electron-rich dienes and electron-poor dienophiles, the reaction with ozone operates fastest with electron-rich alkenes.
Note 5. Another 1,3-dipolar cycloaddition, this time between a carbonyl oxide and a carbonyl. For some fairly conclusive evidence that this step is a concerted cycloaddition and not a two-step series of 1,2-addition reactions snapping shut to give a five-membered ring, see [ref]. This recombination step is stereoselective and dependent on starting alkene geometry.
Note 6. Ozonides are not generally a class of compound you would want to work with, but the naturally occurring ozonide arteminisin is a known antimalarial drug. The ozonide functional group is key to its activity. Other derivatives of arteminisin have been developed as drug candidates.
For a short, fun article on developments on this field, see: “Ozonides as Drugs: What Will They Think of Next?” by Derek Lowe.
Quiz Yourself!
Become a MOC member to see the clickable quiz with answers on the back.
Become a MOC member to see the clickable quiz with answers on the back.
Become a MOC member to see the clickable quiz
Become a MOC member to see the clickable quiz with answers on the back.
Become a MOC member to see the clickable quiz with answers on the back.
Become a MOC member to see the clickable quiz with answers on the back.
(Advanced) References and Further Reading
A fun article on the history of the discovery of ozone written by Mordecai B. Rubin is found here, from the Bulletin of Chemical History. [PDF]
- Ueber die Einwirkung des Ozons auf organische Verbindungen
Harries
Just. Lieb. Ann. Chem. 1905, 343 (2-3), 311-344
DOI: 10.1002/jlac.19053430209
The first paper describing the oxidative cleavage of unsaturated compounds with ozone in solution. - OZONE
I. Smith, F. L. Greenwood, and O. Hudrlik
Org. Synth. 1946 26, 63
DOI: 10.15227/orgsyn.026.0063
This procedure from Organic Syntheses, a source of reliable, reproducible and independently tested organic chemistry laboratory experimental procedures, provides a detailed explanation of how to build a laboratory ozonizer. - The Preparation of Aldehydes, Ketones, and Acids by Ozone Oxidation
Albert L. Henne and Philip Hill
Journal of the American Chemical Society 1943 65 (5), 752-754
DOI: 1021/ja01245a003
This paper shows that carboxylic acids are formed in good yields from aldehydes when the ozonolysis reaction mixture is worked up in the presence of excess hydrogen peroxide. - Notes- A Convenient Method for Reduction of Hydroperoxide Ozonation Products
Knowles and Q. Thompson
The Journal of Organic Chemistry 1960 25 (6), 1031-1033
DOI: 10.1021/jo01076a044
Although the current practice is to use dimethyl sulfide in a reductive ozonolysis workup, trimethyl phosphite can also be used, as this paper from Nobel Laureate W. S. Knowles demonstrates. - OZONOLYTIC CLEAVAGE OF CYCLOHEXENE TO TERMINALLY DIFFERENTIATED PRODUCTS: METHYL 6-OXOHEXANOATE, 6,6-DIMETHOXYHEXANAL, METHYL 6,6-DIMETHOXYHEXANOATE
Ronald E. Claus and Stuart L. Schreiber
Org. Synth. 1986, 64, 150
DOI: 10.15227/orgsyn.064.0150
This procedure in Organic Syntheses demonstrates how ozonolysis can be used to quickly generate differentiated bifunctional compounds. - Mechanism of Ozonolysis
Dr. Rudolf Criegee
Angew. Chem. Int. Ed. 1975, 14 (11), 745-752
DOI: 10.1002/anie.197507451
This is an account by Prof. Rudolf Criegee on work done towards determining the mechanism of ozonolysis. Criegee himself carried out extensive work in this area – the ‘Criegee intermediate’ in ozonolysis is named after him.The following papers are further mechanistic studies on ozonolysis: - Formation and Structure of Ozonides
Robert L. KuczkowskiAccounts of Chemical Research 1983 16 (2), 42-47
DOI: 10.1021/ar00086a002
A good overview on evidence for the the various steps of the ozonolysis reaction. - New evidence in the mechanism of ozonolysis of olefins
Klopman and C. M. Joiner
Journal of the American Chemical Society 1975 97 (18), 5287-5288
DOI: 10.1021/ja00851a049 - Mechanism of ozonolysis. (a) Microwave spectra, structures, and dipole moments of propylene and trans-2-butene ozonides. (b) Orbital symmetry analysis
Robert P. Lattimer, Robert L. Kuczkowski, and Charles W. Gillies
Journal of the American Chemical Society 1974 96 (2), 348-358
DOI:1021/ja00809a006 - Stereospecificity in ozonide and cross-ozonide formation
Nathan L. Bauld, James A. Thompson, Charles E. Hudson, and Philip S. BaileyJournal of the American Chemical Society 1968 90 (7), 1822-1830
DOI: 10.1021/ja01009a026
- Ozonolysis. X. Molozonide as an intermediate in the ozonolysis of cis- and trans-alkenes
Lois J. Durham and Fred L. Greenwood
The Journal of Organic Chemistry 1968 33 (4), 1629-1632
DOI: 10.1021/jo01268a068
Evidence for the formation of a molozonide, which decomposes at -130°C (cis) or -100°C (trans) depending on the stereochemistry of the alkene.
In case of oxidative ozonolysis of terminal alkene of the form =CH2, will the product be HCOOH or CO2?
Formic acid will be oxidized to CO2 in the presence of H2O2.
Thank you for your reply! I tried to draw the mechanism for the oxidative work up of ozonolysis of tetrasubstituted alkene with H2O2. After the ozonide being cleaved into a ketone and a Criegee intermediate, I couldn’t use water to do proton transfer since there’s no H on the carbon, so I tried H2O2 and it seemed like there would be an equivalent of O2 released, but I’m not sure if I’m right. (I saw an article on Chemistry LibreTexts and it says the product would be 2 ketones.)
I have a question about the oxidative workup. What if I use tetrasubstituted alkene in the reaction?
I’ve seen the mechanism of both reductive and oxidative workup in the article but it seems like only the reductive one is possible because the extra oxygen in ozonide can leave with DMS, while in the oxidative one there’s one more oxygen needed to form the OH in the carboxylic acids. Now in the case of tetrasubstituted alkene, there’re no hydrogen to leave for the carbon to allow one more OH group on it. Is it right that I can’t use oxidant to workup the ozonide of tetrasubstituted alkene?
You could perform the workup using either using reductive conditions (best) or oxidative. Usual procedure is just to add dimethylsulfide at low temperature to destroy excess ozonide and then let it warm to room temperature.
Thank you for your suggestion, that is certainly something that might work. I should have clarified that my problem involved a cyclic tetrasubstituted alkene and an internal disubstituted alkene. I want the less substituted alkene to be cleaved and the tetrasubstituted one to be left alone. Will my aforementioned dihydroxylation followed by cleavage of the vicinal diol work?
Yes, I would give it a shot. If you have access to the Sharpless AD-Mix that would be a good thing to try since the bulky ligands should keep your osmium away from the bulkier alkene.
See here:
https://hwpi.harvard.edu/files/myers/files/23-sharpless_asymmetric_dihydroxylation_reaction.pdf
It is my understanding that ozonolysis favours more substituted alkenes. Are there any reactions that favours the cleavage of less substituted alkenes? I think dihydroxylation favours less substituted alkenes, the resulting vicinal diols can then be cleaves with sodium periodate. However, I am not certain if my thinking is correct, if I’m wrong are there any other
methods?
Good question. You are indeed correct that greater alkene substitution favors a higher rate of ozonolysis,
Is your problem concerning selectivity on a molecule with multiple alkenes? Is this something that could be avoided through, say, a later-stage reduction of an alkyne to a cis or trans alkene (alkynes are generally slow to cleave with ozonolysis).
Ozonolysis of 3 methylbut-1-ene in the presence of H202
One of the products is methanal my note here in textbook concert it into CO2
Pls why
And not methanoic acid
Methanal (formaldehyde) is quickly oxidized by H2O2 to give carbonic acid which itself loses water to give CO2 (carbon dioxide)
What happens if you run this reaction in
1. O3
—–>
2. Me2S, H2O
… ?
Does the H2O change anything, as compared to the reaction without H2O?
No, it should not. The only thing water might do is form the hydrate of the aldehyde/ketone, which is reversible anyway.
What if we have one mole of an alkene with two double bond and only one mole of ozone?
Hi i was just wondering what the mechanism would be for example if i started with cyclohexene and used 03 and Me2S
The mechanism would be identical to the generic reductive workup example. The product would be a six carbon chain with an aldehyde at each end; hexanedial
What do u mean sir
What about Ozonolysis on O-xylene? I assume the reaction would take place at all of the alkenes? If this is the case, would it require 3 equivalents?
Ozonolysis on an aromatic molecule is generally a no-go, except under forcing conditions.
I aspire to reach your level of orgo proficiency. Like for real, reading your answers to these questions is awesome. Orgo 2 final coming up for me
hi i am in 11th grade and i got a question where i was given the final products that were obtained whilst the reactants underwent ozonolysis. how do i find out the reactant(IUPAC name) if the product is given. Pls help
You need to work backwards. Analyze the bonds formed and broken in the forward direction of ozonolysis. Now apply the same pattern, but in reverse.
What happens if a compound containing both double and triple bond is introduced to ozonolysis?which one will get reduced and why?
Alkynes are considerably more resistant towards ozonolysis than alkenes. It’s possible to selectively cleave alkenes in the presence of alkynes.
I found a question
Ozonolysis of cyclohexanone using Zn as a catalyst yields which products ?
Thanks !
Zn is used in the workup step, not with ozonolysis itself. Your product would be hexanedial.
What is the difference between oxidative ozonolysis and reductive ozonolysis
And does their products area same or different
It’s “oxidative workup” and “reductive workup”. The difference is discussed in the article. Oxidative workup will convert aldehydes to carboxylic acids.
What is the difference for ozonolysis by zinc and water
Ozonolysis is not done with zinc and water. Ozonolysis is done with ozone. The workup is done with zinc and water.
Give me mechanism for types of oxidative and reductive ozonolysis
Oxidative workup and reductive workup. There’s a comprehensive treatment in the Reaction Guide.
What is the pros and cons using ozonolysis?
Pros: works well at gram-scale, and ease of workup. Basically add reductant at -78 and let warm, followed by concentration. Cons: poor at explorative scale (e.g. 100 mg and less) where it’s more convenient to use OsO4/NaIO4 (Johnson-Lemieux cleavage).
when comparing the ozonolysis reaction to the KMnO4 (hot) reaction, why is it that ozonolysis doesn’t form carboxylic acids on alkenes but will on alkynes? KMnO4 will form carboxylic acids on both alkynes and alkenes.
Ozonolysis cleaves all C-C bonds and converts to C-O bonds. In an alkyne three bonds are cleaved, and instead of forming C(triple bond)O, which would have a positive formal charge at oxygen, one forms a carboxylic acid instead.
If there are multiple alkenes within the original molecule but there is only one equivalent of O3, how do you determine which alkene to cleave and which to leave as is? Thanks!
Hi – good question!
Generally, the more highly substituted the alkene, the more electron rich it is, and the more reactive it will be towards O3 (sterics are not a significant factor in most cases).
It’s possible to selectively ozonize an alkene assuming there is a significant difference in substitution pattern.
For instance it’s possible to selectively ozonize a tetrasubstituted alkene in the presence of a disubstituted alkene. The trick is to use a dye as an indicator (an example is Sudan Red) that is intermediate in reactivity between the alkene you are trying to ozonize and the less reactive alkene. When the dye starts getting chewed up you will notice a color change and you can stop the reaction.
Benzene ring breaks on ozonolysis in all cases
Absolutely not. Only under extremely forcing conditions.
does ozonolysis take place at any temperature please ?? thanx
Usually -78 degrees, temperature of dry ice-acetone bath.
when there is a cyclopentene attached to benzene ring, and ozonolysis is carried out then will the reaction take place only on pentene…? why does that happen..?
Yes, benzene is relatively inert to ozonolysis unless it is extremely electron rich (e.g. has alkoxy groups)
well from what reactant by ozonlysis can we form cyclohexanone???
You’ll need to learn how to think backwards.
Cyclohexane double bounded to CH2 should give you cyclohexanone
With formaldehyde as by product
Correct?
Yes, methylenecyclohexane is the simplest precursor that will work.
The acid is needed to hydrolise the Carbon Oxygen bond in the Ozonide intermediate
Now ‘aldehyd’ is still written under this carboxylic acid.
This has been fixed. Thank you
I believe there is a typo- in the last mechanism, it is described c=o,-oh to be an “aldehyde” where it should be a carboxylic acid? (In oxidative workup, where the H is replaced with OH).
Ah yes, it should be a carboxylic acid. Will fix. Thanks!
I had a question where it was a reaction map for turning a generic terminal alkyne into a generic aldehyde. I was going for using hydrogenization in the presence of Lindlar’s and ozonolysis.
In the answer provided (one of many ways), the alkyne underwent ozonolysis with ozone followed by zinc in acid (h3O+) what would the purpose of the acid be?
The acid helps to break up the intermediate ozonide – it protonates one of the oxygens and makes it a better leaving group, in a way similar to how acid helps to cleave acetals.