Home / Electrophilic Aromatic Substitution – The Mechanism
Reactions of Aromatic Molecules
Electrophilic Aromatic Substitution – The Mechanism
Last updated: February 28th, 2025 |
Electrophilic Aromatic Substitution: The Mechanism
- Electrophilic aromatic substitution (EAS) reactions proceed through a two-step mechanism.
- In the first step, the aromatic ring, acting as a nucleophile, attacks an electrophile (E+).
- This is the slow (rate-determining) step since it disrupts aromaticity and results in a carbocation intermediate.
- In the second (fast) step a C-H bond is deprotonated to re-form a C-C pi bond, restoring aromaticity.
- The first step resembles attack of an alkene on H+, and the second step resembles the second step of the E1 reaction. The end result is substitution. (Break C-H, form C-E).
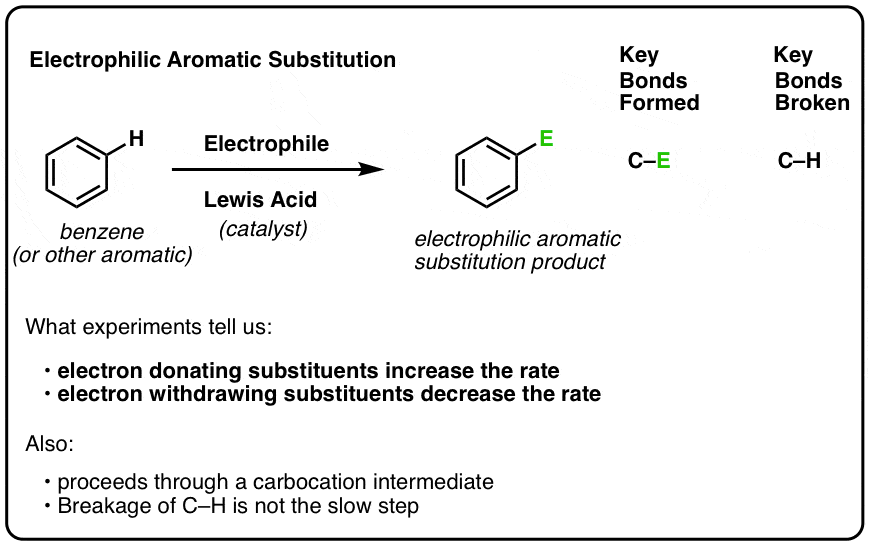
Table of Contents
- Electrophilic Aromatic Substitution Mechanism, Step 1: Attack of The Electrophile (E) By a Pi-bond Of The Aromatic Ring
- Electrophilic Aromatic Substitution Mechanism, Step 2: Deprotonation Of The Tetrahedral Carbon Regenerates The Pi Bond
- Putting Two Steps Together: The General Mechanism
- The Reaction Energy Diagram of Electrophilic Aromatic Substitution
- Beyond Benzene: Formation Of Ortho, Meta, and Para Disubstituted Benzenes
- EAS On Monosubstituted Benzenes: The Distribution Of Ortho, Meta and Para Isomers Is NOT Random
- Notes
- Quiz Yourself!
- (Advanced) References and Further Reading
1. Electrophilic Aromatic Substitution Mechanism, Step 1: Attack of The Electrophile (E) By a Pi-bond Of The Aromatic Ring
Last post in this series on reactions of aromatic groups we introduced activating and deactivating groups in Electrophilic Aromatic Substitution (EAS). We learned that electron-donating substituents on the aromatic ring increase the reaction rate and electron-withdrawing substituents decrease the rate. [In the fine print, we also mentioned that evidence strongly suggests that the reaction proceeds through a carbocation intermediate, and that breakage of C-H is not the slow step.]
Having established these facts, we’re now ready to go into the general mechanism of this reaction.
It’s a two-step process.
The good news is that you’ve actually seen both of the steps before (in Org 1) but as part of different reactions!
The first step of electrophilic aromatic substitution is attack of the electrophile (E+) by a pi bond of the aromatic ring. [Note: the identity of the electrophile E is specific to each reaction, and generation of the active electrophile is a mechanistic step in itself. We’ll cover the specific reactions next. This post just covers the general framework for electrophilic aromatic substitution].
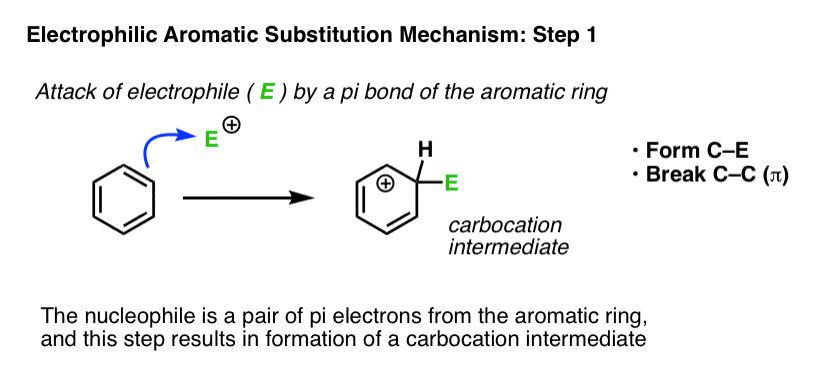
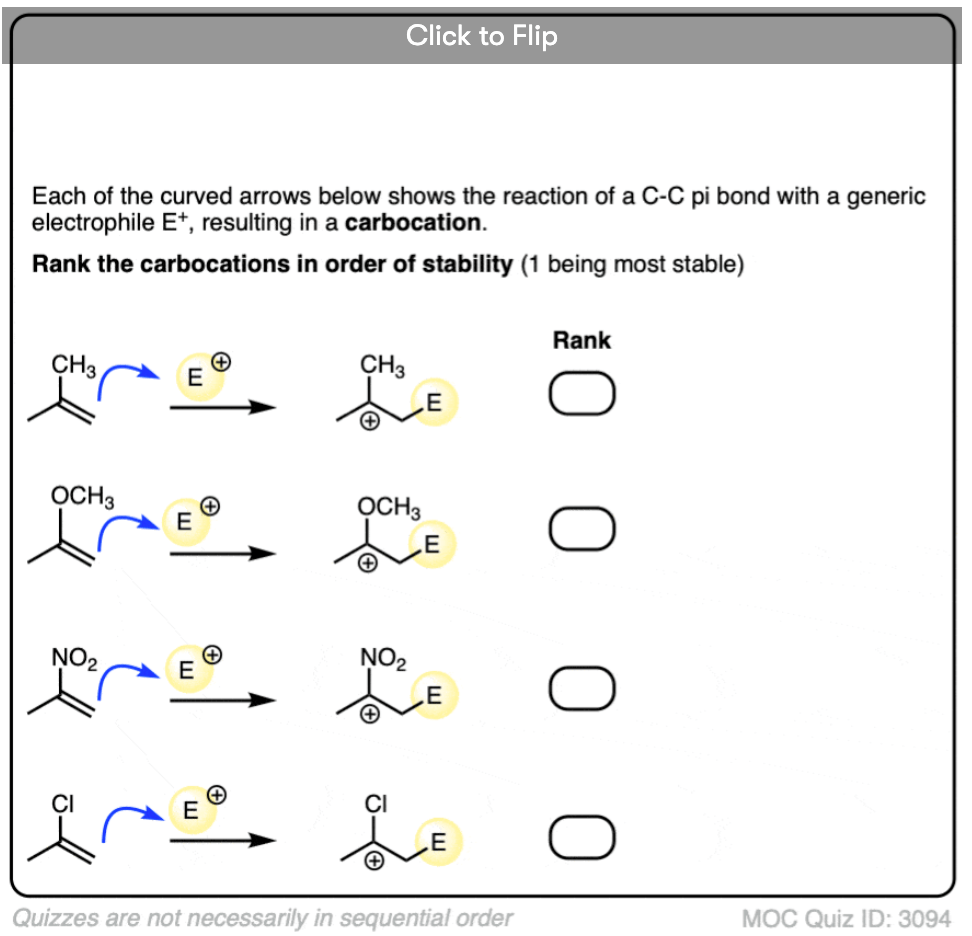
Where have we seen this type of step before? In the chapter on alkenes, we saw a whole series of reactions of pi bonds with electrophiles that generate a carbocation. A common example is the reaction of alkenes with a strong acid such as H-Cl, leading to formation of a carbocation. The reaction above is the same step, only applied to an aromatic ring.
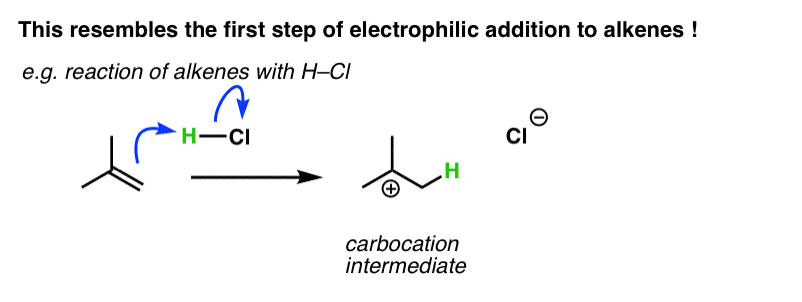
You might recall that the second step of addition of HCl to alkenes is the attack of Cl on the carbocation, generating a new C-Cl bond. That’s not what happens in electrophilic aromatic substitution. [Note 1]
2. Electrophilic Aromatic Substitution Mechanism, Step 2: Deprotonation Of The Tetrahedral Carbon Regenerates The Pi Bond
The second step of electrophilic aromatic substitution is deprotonation. This breaks C–H and forms C–C (π), restoring aromaticity. You may recall that this is strongly favored – the resonance energy of benzene is about 36 kcal/mol. [This is the type of phenomenon chemists like to call a “thermodynamic sink” – over time, the reaction will eventually flow to this final product, and stay there. ]
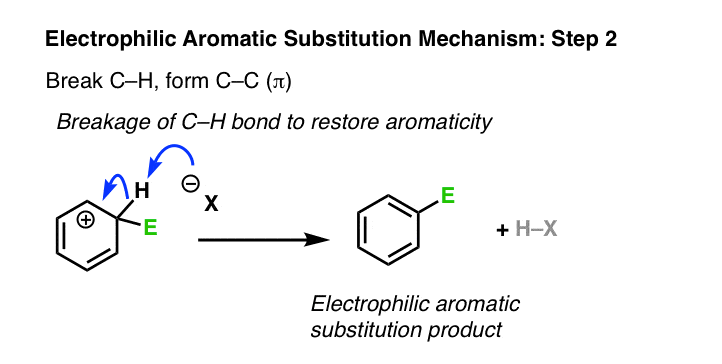
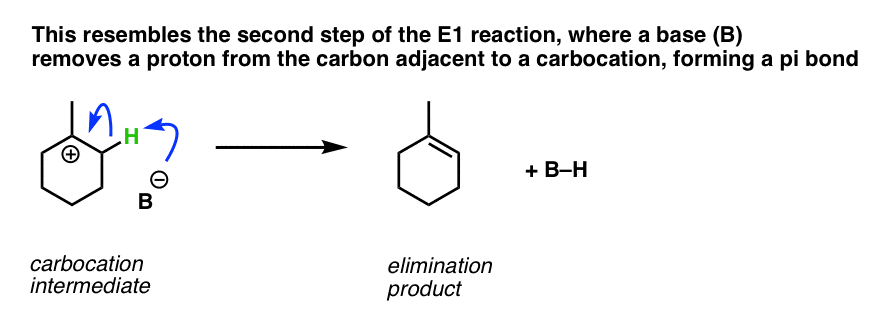
Have we seen this type of step before? Yes – it’s essentially the second step of the E1 reaction, (after loss of a leaving group) where a carbon adjacent to a carbocation is deprotonated, forming a new C-C pi bond.
Just as in the E1, a strong base is not required here. A halogen atom (such as Cl– ) will usually suffice, as will any number of other weak bases, such as H2O. The exact identity of the base depends on the reagents and solvent used in the reaction.
3. Putting Two Steps Together: The General Mechanism
Let’s combine both steps to show the full mechanism. Again, we won’t go into the details of generating the electrophile E, as that’s specific to each reaction.
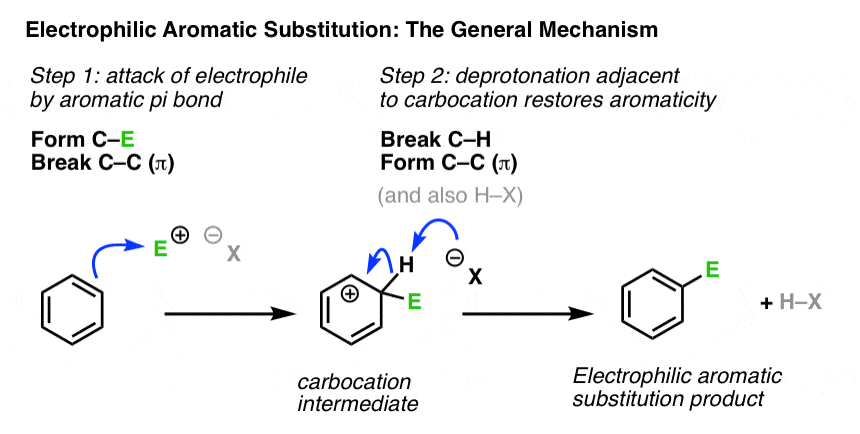
Note that attack could have occurred at any one of the six carbons of benzene and resulted in the same product.
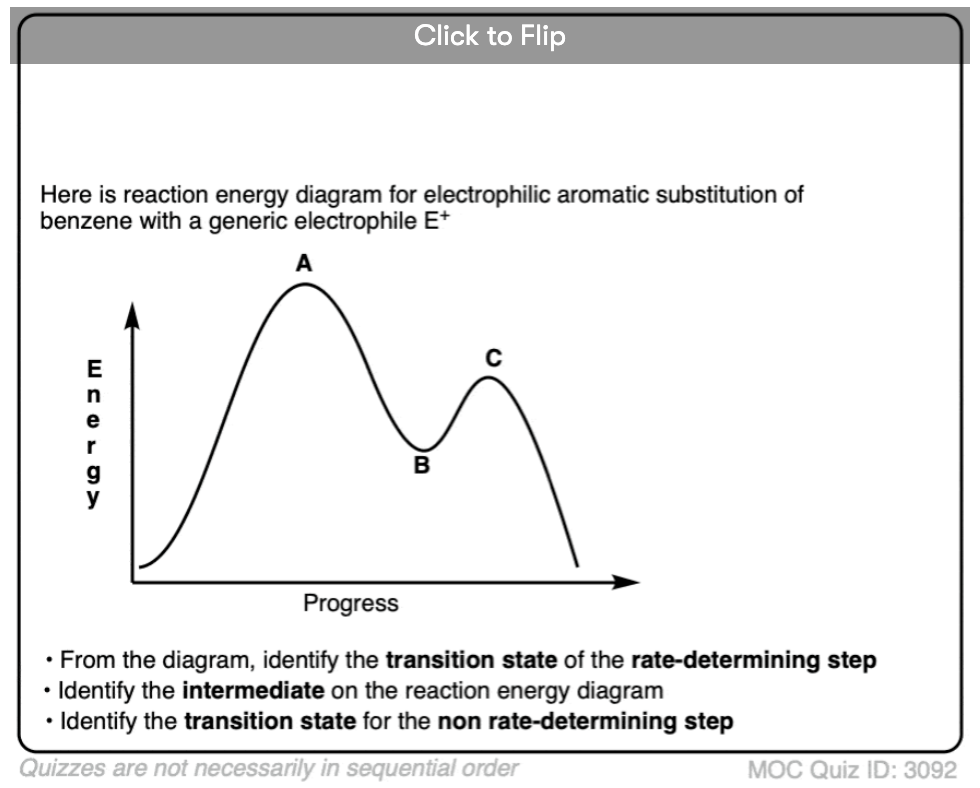
4. The Reaction Energy Diagram of Electrophilic Aromatic Substitution
What might the reaction energy diagram of electrophilic aromatic substitution look like?
First, the overall appearance is determined by the number of transition states in the process.
Recall that transition states always have partial bonds and are at the “peaks” of a reaction energy diagram, and intermediates such as carbocations are in the “valleys” between peaks. Intermediates can be observed and isolated (at least in theory); in contrast, transition states have a lifetime of femtoseconds, and although they may fleetingly be observed in certain cases, they can never be isolated.
Electrophilic aromatic substitution has two steps (attack of electrophile, and deprotonation) which each have their own transition state. There is also a carbocation intermediate. This means that we should have a “double-humped” reaction energy diagram.
Second, the relative heights of the “peaks” should reflect the rate-limiting step.
What’s the slow step? In other words, which of the two steps has the highest activation energy?
One clue is to measure the effect that small modifications to the starting material have on the reaction rate.
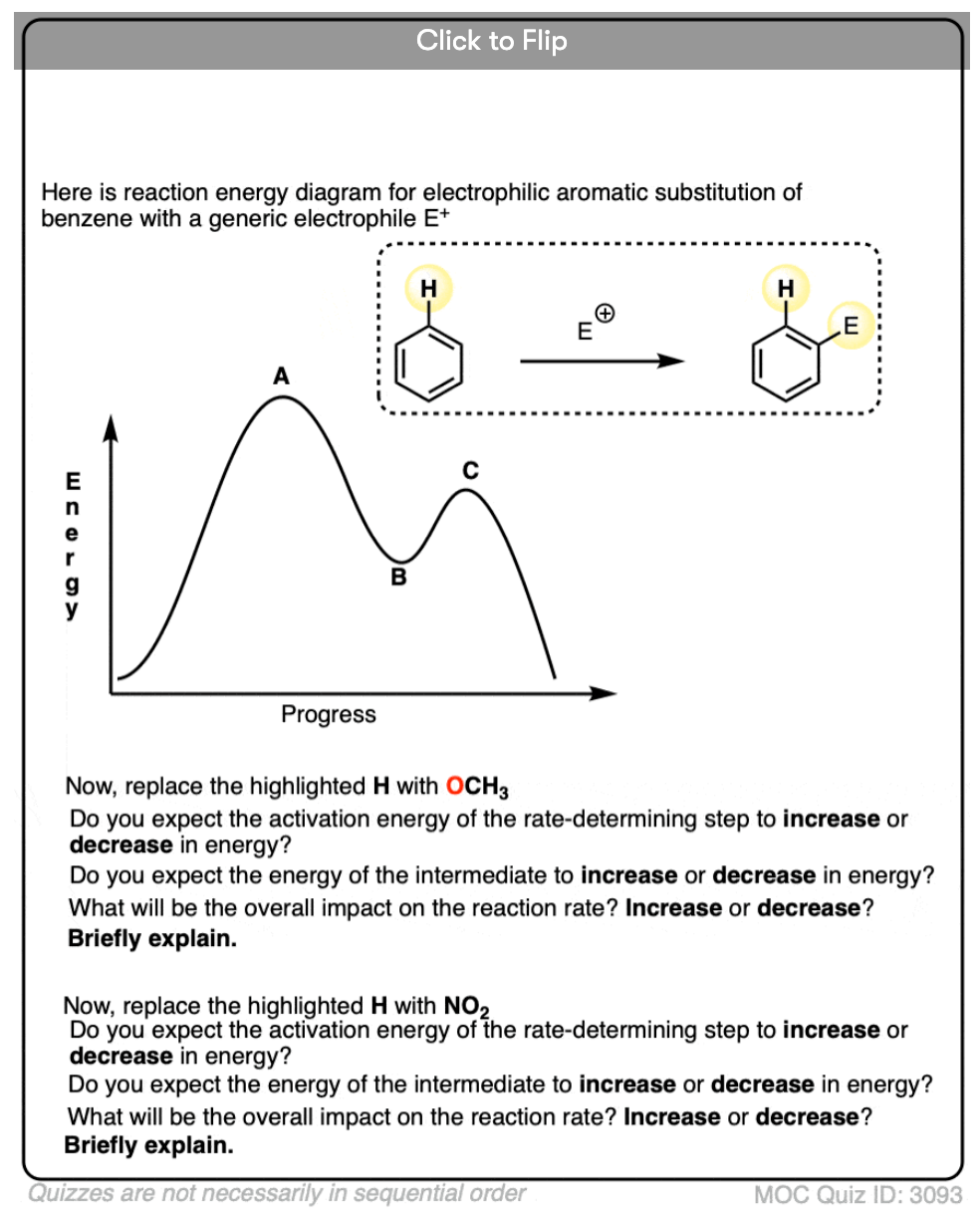
We showed in the last post that electron-donating substitutents increase the rate of reaction (“activating”) and electron-withdrawing substituents decrease the rate of reaction (“deactivating”). [Conversely, substitution of hydrogen for deuterium has very little effect on the reaction rate, which leads us to conclude that the second step is not rate-determining. ]
Since electron-donating and electron-withdrawing substitutents affect the nucleophilicity of the pi bond (through pi-donation and pi-acceptance) as well as the stability of the intermediate carbocation, the logical conclusion is that attack on the electrophile (step 1) is the rate-determining step. We therefore should depict it with the higher “hump” in our reaction energy diagram, representing its higher activation energy.
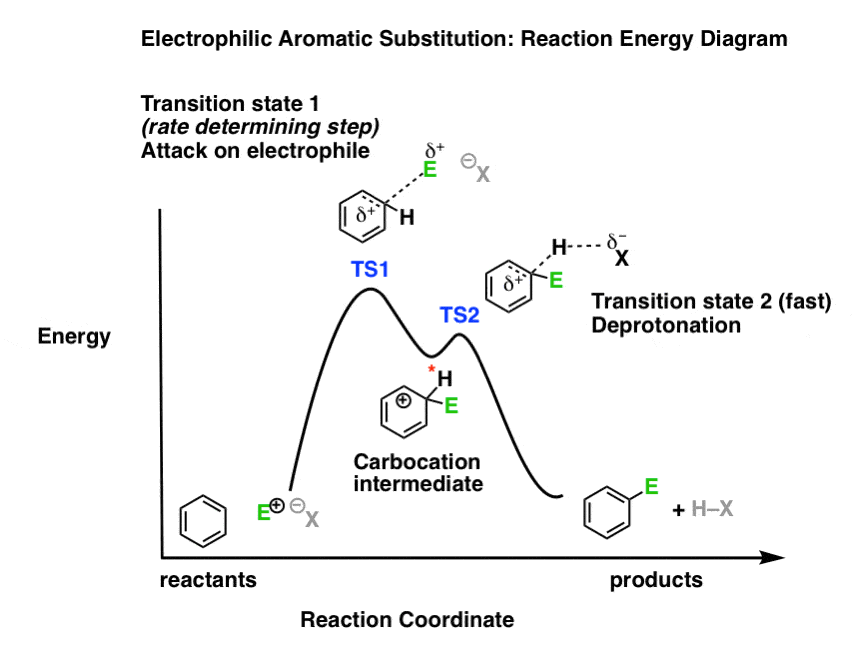
Note that this reaction energy diagram is not to scale and is more of a sketch than anything else. A truly accurate reaction energy diagram can be modelled if one had accurate energies of the transition states and intermediates, which is sometimes available through calculation.
5. Beyond Benzene: Formation Of Ortho, Meta, and Para Disubstituted Benzenes
So that’s all there is to electrophilic aromatic substitution? Yes and no.
Yes, this addresses electrophilic aromatic substitution for benzene.
But, as you’ve no doubt experienced, small changes in structure can up the complexity a notch.
Imagine we start not with benzene, but with a mono-substituted derivative, such as methylbenzene (toluene).
What are the possible products of electrophilic aromatic substitution on a mono-substituted benzene derivative?
Unlike with benzene, where only one EAS product is possible due to the fact that all six hydrogens are equivalent, electrophilic aromatic substitution on a mono-substituted derivative can yield three possible products: the 1,2- isomer (also called “ortho“), the 1,3-isomer (“meta“) and the 1,4-isomer (“para“).
If we assume that the reaction obeys the laws of statistics, we might therefore expect that the product distribution should be 40% ortho. 40% meta, and 20% para.
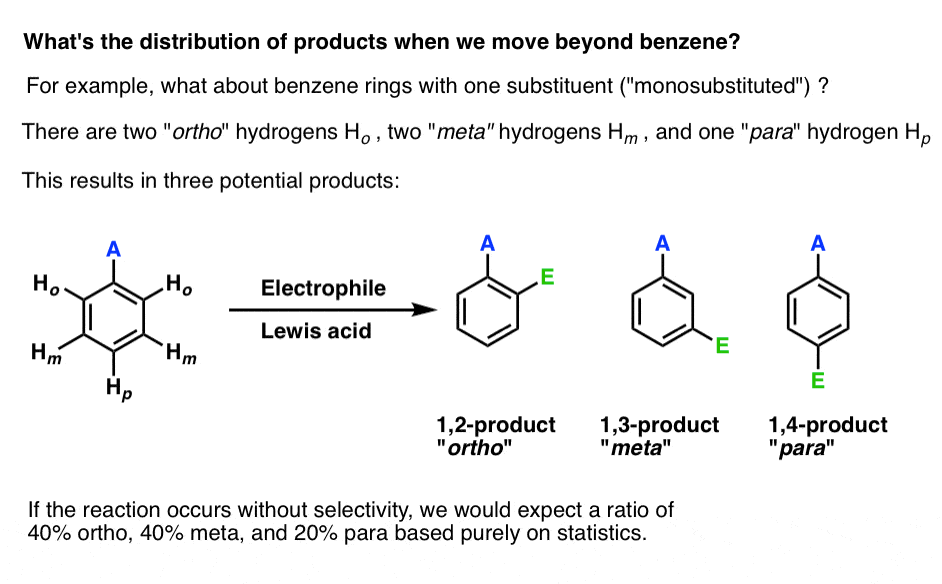
So is that what happens?
No! Two important examples are illustrative.
6. EAS On Monosubstituted Benzenes: The Distribution Of Ortho, Meta and Para Isomers Is NOT Random!
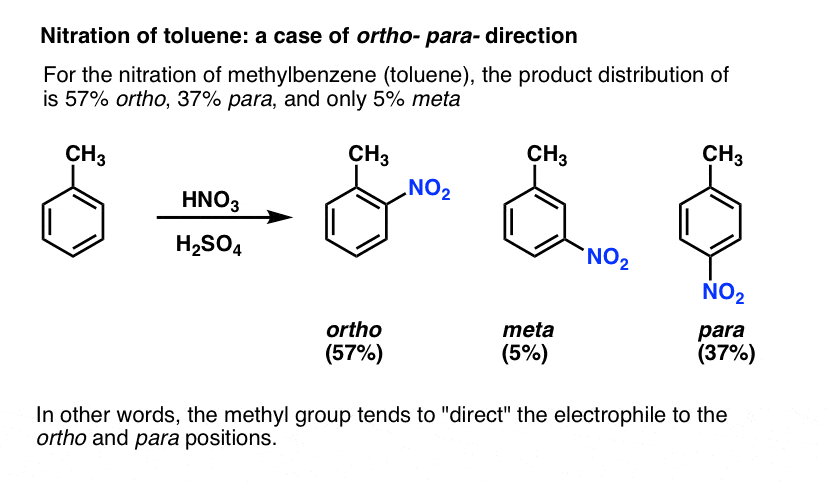
In the nitration of toluene, the product distribution is far from statistical. We get much less meta (5%) than expected, and more ortho (57%) and para (37%) than expected.
In this sense we can say that the methyl group tends to act as an ortho- para- director: it “directs” the electrophile to these positions at the expense of the meta position.
Is this the case for all substituents? No.
In the nitration of nitrobenzene, the opposite result is obtained. Much less ortho and para is produced than expected, and the meta product is major (93%).
In this case the nitro group is said to be acting as a meta- director.
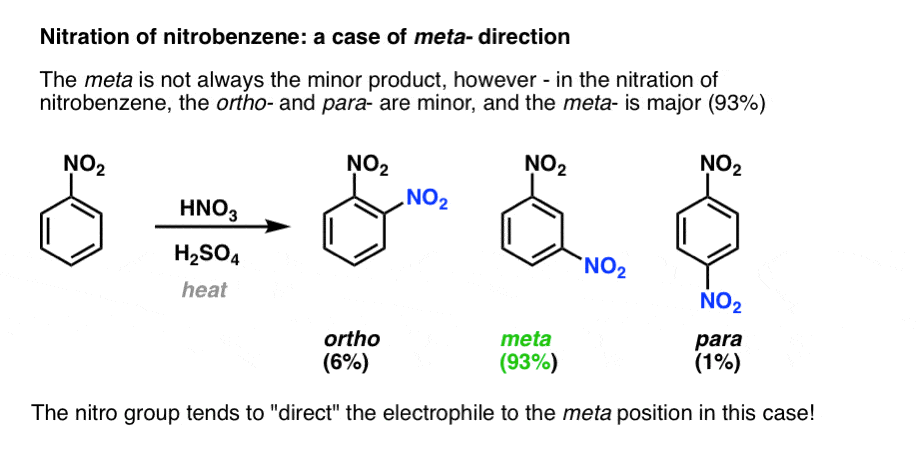
Substituents on benzene tend to fall into one of two categories: ortho–para directors, or meta directors.
If you’re sharp, you might have already made an intuitive leap: the ortho- para- directing methyl group is an activating group, and the meta- directing nitro group is deactivating. So, therefore, are all activating groups ortho- para- directors and all deactivating groups meta- directors?
It’s a good guess – and almost accurate! The fly in the bourbon is the halogens (F, Cl, Br, I) which are deactivating ortho-para directors.
Why? What leads some substituents to be ortho-para directors, and others to be meta-directors?
That’s going to have to wait until the next post for a full discussion. But here’s a hint: it has to do with our old friend, “pi-donation”.
Thanks to Mattbew Knowe for valuable assistance with this post.
Notes
Related Articles
- Electrophilic Aromatic Substitutions (1) – Halogenation of Benzene
- Electrophilic Aromatic Substitutions (2) – Nitration and Sulfonation
- Aromatic Synthesis (3) – Sulfonyl Blocking Groups
- EAS Reactions (3) – Friedel-Crafts Acylation and Friedel-Crafts Alkylation
- Intramolecular Friedel-Crafts Reactions
- Aromatic Reactions and Synthesis Practice (MOC Membership)
- Understanding Ortho, Para, and Meta Directors
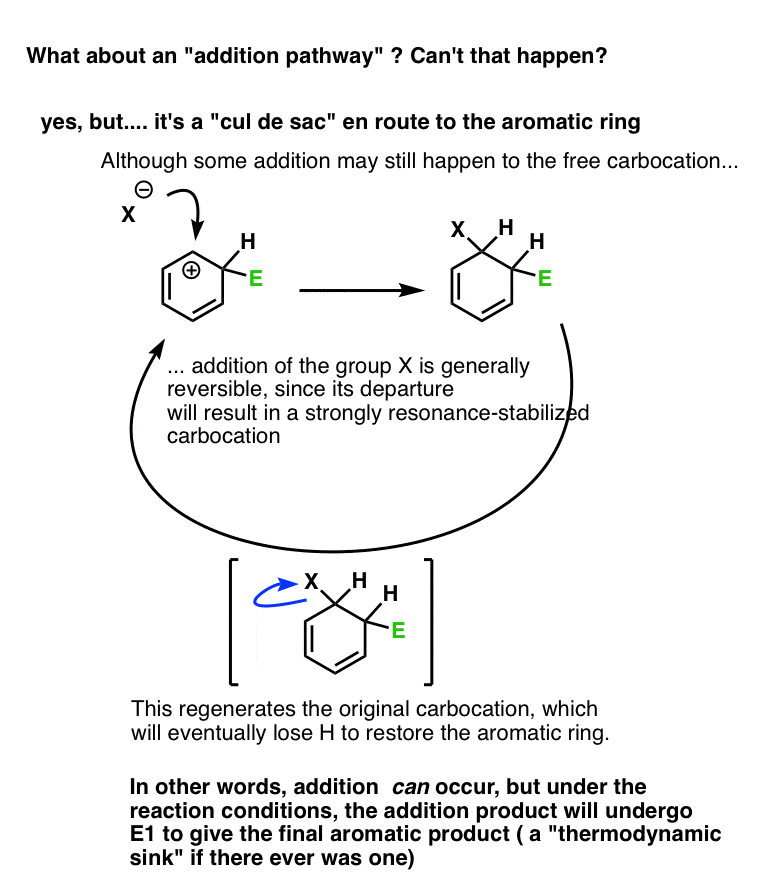
Note 1 – Why can’t the counterion attack the aromatic ring carbocation? Does that happen?
Yes, but it’s a dead end.
Let’s say we form the carbocation, and it’s attacked by a weak nucleophile (which we’ll call X).
This gives us the addition product.
However, it’s rarely a very stable product. X is typically a weak nucleophile, and therefore a good leaving group. Furthermore, loss of the leaving group will result in a highly resonance-stabilized carbocation. (Think of the first step in the SN1 or E1 reaction).
This would re-generate the carbocation, which could then undergo deprotonation to restore aromaticity. Once that aromatic ring is formed, it’s not going anywhere. : – )
To make a long story short, yes, addition could occur, but the addition product will eventually undergo E1 to form the aromatic product.
(figure below)
Quiz Yourself!
Become a MOC member to see the clickable quiz with answers on the back.
Become a MOC member to see the clickable quiz with answers on the back.
Become a MOC member to see the clickable quiz with answers on the back.
Become a MOC member to see the clickable quiz with answers on the back.
Become a MOC member to see the clickable quiz with answers on the back.
(Advanced) References and Further Reading
The EAS mechanism covers a variety of reactions – Friedel-Crafts substitutions, halogenation, nitration, and many others.
- A Quantum Mechanical Investigation of the Orientation of Substituents in Aromatic Molecules
G. W. Wheland
Journal of the American Chemical Society 1942, 64 (4), 900-908
DOI: 10.1021/ja01256a047
This discusses the structure of the arenium ion that gets formed in EAS reactions, also known as the s-complex or Wheland intermediate, after the author here who first proposed it. - A Quantitative Treatment of Directive Effects in Aromatic Substitution
Leon M. Stock, Herbert C. Brown
Phys. Org. Chem. 1963, 1, 35-154
DOI: 10.1016/S0065-3160(08)60277-4
This is a very comprehensive review for its time, summarizing work on directing effects in EAS (e.g. determining which groups are o/p-directing vs. meta-directing, and to what extent they direct/deactivate). - Aromatic substitution. XXVIII. Mechanism of electrophilic aromatic substitutions
George A. Olah
Acc. Chem. Res., 1971, 4 (7), 240-248
DOI: 10.1021/ar50043a002
An account by Prof. Olah on the work he had carried out studying the mechanism of various types of electrophilic aromatic substitution reactions – nitration, halogenation, as well as Friedel-Crafts acylation and alkylation. - Aromatic substitution. XXXVI. Aluminum trichloride and antimony pentafluoride catalyzed Friedel-Crafts alkylation of benzene and toluene with esters and haloesters
George A. Olah and Jun Nishimura
Journal of the American Chemical Society 1974, 96 (7), 2214-2220
DOI: 10.1021/ja00814a035
In this case, carboxylic esters are not studied (as those would lead to acylation rather than alkylation). This covers other types of esters in Friedel-Crafts alkylation: alkyl chlorosulfites, arenesulfinates, tosylates, chloro- and fluorosulfates, trifluoromethanesulfonates (triflates), pentafluorobenzenesulfonates, and trifluoroacetates. - Aromatic substitution. XXXVII. Stannic and aluminum chloride catalyzed Friedel-Crafts alkylation of naphthalene with alkyl halides. Differentiation of kinetically and thermodynamically controlled product compositions, and the isomerization of alkylnaphthalenes
George A. Olah and Judith A. Olah
Journal of the American Chemical Society 1976, 98 (7), 1839-1842
DOI: 10.1021/ja00423a032
This is a similar paper by Prof. Olah and his wife, Judith Olah, on the mechanism of Friedel-Crafts alkylation, except using naphthalene instead of benzene. Naphthalene is different in that there are two sites for monosubstitution – the a and b positions. - Stable carbocations. CLXX. Ethylbenzenium ions and the heptaethylbenzenium ion
George A. Olah, Robert J. Spear, Guisseppe Messina, and Phillip W. Westerman
Journal of the American Chemical Society 1975, 97 (14), 4051-4055
DOI: 1021/ja00847a031
This paper discusses the characterization of benzenium ions, which are intermediates in EAS, and the characterization of the heptaethylbenzenium ion, which is a stable species because it lacks a proton and therefore eliminates with difficulty. - The Anomalous Reactivity of Fluorobenzene in Electrophilic Aromatic Substitution and Related Phenomena
Joel Rosenthal and David I. Schuster
Journal of Chemical Education 2003, 80 (6), 679
DOI: 10.1021/ed080p679
A very interesting paper, suitable for curious undergrads, and discusses something that most practicing organic chemists will know empirically – fluorobenzene is almost as reactive as benzene in EAS or Friedel-Crafts reactions, which is counterintuitive when one considers electronic effects. - Electrophilic Aromatic Substitution: New Insights into an Old Class of Reactions
Boris Galabov, Didi Nalbantova, Paul von R. Schleyer, and Henry F. Schaefer, III
Accounts of Chemical Research 2016, 49 (6), 1191-1199
DOI: 10.1021/acs.accounts.6b00120
The late Prof. P. v. R. Schleyer was a giant in Physical Organic chemistry, and this paper, published posthumously, covers work done towards the end of his life in re-determining the mechanism of EAS. - Unified Mechanistic Concept of Electrophilic Aromatic Nitration: Convergence of Computational Results and Experimental Data
Pierre M. Esteves, José Walkimar de M. Carneiro, Sheila P. Cardoso, André H. Barbosa, Kenneth K. Laali, Golam Rasul, G. K. Surya Prakash, and George A. Olah
Journal of the American Chemical Society 2003, 125 (16), 4836-4849
DOI: 10.1021/ja021307w
This is the grand-daddy paper on nitration, summarizing a lifetime’s worth of work on the subject.
00 General Chemistry Review
01 Bonding, Structure, and Resonance
- How Do We Know Methane (CH4) Is Tetrahedral?
- Hybrid Orbitals and Hybridization
- How To Determine Hybridization: A Shortcut
- Orbital Hybridization And Bond Strengths
- Sigma bonds come in six varieties: Pi bonds come in one
- A Key Skill: How to Calculate Formal Charge
- The Four Intermolecular Forces and How They Affect Boiling Points
- 3 Trends That Affect Boiling Points
- How To Use Electronegativity To Determine Electron Density (and why NOT to trust formal charge)
- Introduction to Resonance
- How To Use Curved Arrows To Interchange Resonance Forms
- Evaluating Resonance Forms (1) - The Rule of Least Charges
- How To Find The Best Resonance Structure By Applying Electronegativity
- Evaluating Resonance Structures With Negative Charges
- Evaluating Resonance Structures With Positive Charge
- Exploring Resonance: Pi-Donation
- Exploring Resonance: Pi-acceptors
- In Summary: Evaluating Resonance Structures
- Drawing Resonance Structures: 3 Common Mistakes To Avoid
- How to apply electronegativity and resonance to understand reactivity
- Bond Hybridization Practice
- Structure and Bonding Practice Quizzes
- Resonance Structures Practice
02 Acid Base Reactions
- Introduction to Acid-Base Reactions
- Acid Base Reactions In Organic Chemistry
- The Stronger The Acid, The Weaker The Conjugate Base
- Walkthrough of Acid-Base Reactions (3) - Acidity Trends
- Five Key Factors That Influence Acidity
- Acid-Base Reactions: Introducing Ka and pKa
- How to Use a pKa Table
- The pKa Table Is Your Friend
- A Handy Rule of Thumb for Acid-Base Reactions
- Acid Base Reactions Are Fast
- pKa Values Span 60 Orders Of Magnitude
- How Protonation and Deprotonation Affect Reactivity
- Acid Base Practice Problems
03 Alkanes and Nomenclature
- Meet the (Most Important) Functional Groups
- Condensed Formulas: Deciphering What the Brackets Mean
- Hidden Hydrogens, Hidden Lone Pairs, Hidden Counterions
- Don't Be Futyl, Learn The Butyls
- Primary, Secondary, Tertiary, Quaternary In Organic Chemistry
- Branching, and Its Affect On Melting and Boiling Points
- The Many, Many Ways of Drawing Butane
- Wedge And Dash Convention For Tetrahedral Carbon
- Common Mistakes in Organic Chemistry: Pentavalent Carbon
- Table of Functional Group Priorities for Nomenclature
- Summary Sheet - Alkane Nomenclature
- Organic Chemistry IUPAC Nomenclature Demystified With A Simple Puzzle Piece Approach
- Boiling Point Quizzes
- Organic Chemistry Nomenclature Quizzes
04 Conformations and Cycloalkanes
- Staggered vs Eclipsed Conformations of Ethane
- Conformational Isomers of Propane
- Newman Projection of Butane (and Gauche Conformation)
- Introduction to Cycloalkanes
- Geometric Isomers In Small Rings: Cis And Trans Cycloalkanes
- Calculation of Ring Strain In Cycloalkanes
- Cycloalkanes - Ring Strain In Cyclopropane And Cyclobutane
- Cyclohexane Conformations
- Cyclohexane Chair Conformation: An Aerial Tour
- How To Draw The Cyclohexane Chair Conformation
- The Cyclohexane Chair Flip
- The Cyclohexane Chair Flip - Energy Diagram
- Substituted Cyclohexanes - Axial vs Equatorial
- Ranking The Bulkiness Of Substituents On Cyclohexanes: "A-Values"
- Cyclohexane Chair Conformation Stability: Which One Is Lower Energy?
- Fused Rings - Cis-Decalin and Trans-Decalin
- Naming Bicyclic Compounds - Fused, Bridged, and Spiro
- Bredt's Rule (And Summary of Cycloalkanes)
- Newman Projection Practice
- Cycloalkanes Practice Problems
05 A Primer On Organic Reactions
- The Most Important Question To Ask When Learning a New Reaction
- Curved Arrows (for reactions)
- Nucleophiles and Electrophiles
- The Three Classes of Nucleophiles
- Nucleophilicity vs. Basicity
- What Makes A Good Nucleophile?
- What Makes A Good Leaving Group?
- 3 Factors That Stabilize Carbocations
- Equilibrium and Energy Relationships
- 7 Factors that stabilize negative charge in organic chemistry
- 7 Factors That Stabilize Positive Charge in Organic Chemistry
- What's a Transition State?
- Hammond's Postulate
- Learning Organic Chemistry Reactions: A Checklist (PDF)
- Introduction to Oxidative Cleavage Reactions
06 Free Radical Reactions
- Bond Dissociation Energies = Homolytic Cleavage
- Free Radical Reactions
- 3 Factors That Stabilize Free Radicals
- What Factors Destabilize Free Radicals?
- Bond Strengths And Radical Stability
- Free Radical Initiation: Why Is "Light" Or "Heat" Required?
- Initiation, Propagation, Termination
- Monochlorination Products Of Propane, Pentane, And Other Alkanes
- Selectivity In Free Radical Reactions
- Selectivity in Free Radical Reactions: Bromination vs. Chlorination
- Halogenation At Tiffany's
- Allylic Bromination
- Bonus Topic: Allylic Rearrangements
- In Summary: Free Radicals
- Synthesis (2) - Reactions of Alkanes
- Free Radicals Practice Quizzes
07 Stereochemistry and Chirality
- Types of Isomers: Constitutional Isomers, Stereoisomers, Enantiomers, and Diastereomers
- How To Draw The Enantiomer Of A Chiral Molecule
- How To Draw A Bond Rotation
- Introduction to Assigning (R) and (S): The Cahn-Ingold-Prelog Rules
- Assigning Cahn-Ingold-Prelog (CIP) Priorities (2) - The Method of Dots
- Enantiomers vs Diastereomers vs The Same? Two Methods For Solving Problems
- Assigning R/S To Newman Projections (And Converting Newman To Line Diagrams)
- How To Determine R and S Configurations On A Fischer Projection
- The Meso Trap
- Optical Rotation, Optical Activity, and Specific Rotation
- Optical Purity and Enantiomeric Excess
- What's a Racemic Mixture?
- Chiral Allenes And Chiral Axes
- Stereochemistry Practice Problems and Quizzes
08 Substitution Reactions
- Nucleophilic Substitution Reactions - Introduction
- Two Types of Nucleophilic Substitution Reactions
- The SN2 Mechanism
- Why the SN2 Reaction Is Powerful
- The SN1 Mechanism
- The Conjugate Acid Is A Better Leaving Group
- Comparing the SN1 and SN2 Reactions
- Polar Protic? Polar Aprotic? Nonpolar? All About Solvents
- Steric Hindrance is Like a Fat Goalie
- Common Blind Spot: Intramolecular Reactions
- Substitution Practice - SN1
- Substitution Practice - SN2
09 Elimination Reactions
- Elimination Reactions (1): Introduction And The Key Pattern
- Elimination Reactions (2): The Zaitsev Rule
- Elimination Reactions Are Favored By Heat
- Two Elimination Reaction Patterns
- The E1 Reaction
- The E2 Mechanism
- E1 vs E2: Comparing the E1 and E2 Reactions
- Antiperiplanar Relationships: The E2 Reaction and Cyclohexane Rings
- Bulky Bases in Elimination Reactions
- Comparing the E1 vs SN1 Reactions
- Elimination (E1) Reactions With Rearrangements
- E1cB - Elimination (Unimolecular) Conjugate Base
- Elimination (E1) Practice Problems And Solutions
- Elimination (E2) Practice Problems and Solutions
10 Rearrangements
11 SN1/SN2/E1/E2 Decision
- Identifying Where Substitution and Elimination Reactions Happen
- Deciding SN1/SN2/E1/E2 (1) - The Substrate
- Deciding SN1/SN2/E1/E2 (2) - The Nucleophile/Base
- SN1 vs E1 and SN2 vs E2 : The Temperature
- Deciding SN1/SN2/E1/E2 - The Solvent
- Wrapup: The Key Factors For Determining SN1/SN2/E1/E2
- Alkyl Halide Reaction Map And Summary
- SN1 SN2 E1 E2 Practice Problems
12 Alkene Reactions
- E and Z Notation For Alkenes (+ Cis/Trans)
- Alkene Stability
- Alkene Addition Reactions: "Regioselectivity" and "Stereoselectivity" (Syn/Anti)
- Stereoselective and Stereospecific Reactions
- Hydrohalogenation of Alkenes and Markovnikov's Rule
- Hydration of Alkenes With Aqueous Acid
- Rearrangements in Alkene Addition Reactions
- Halogenation of Alkenes and Halohydrin Formation
- Oxymercuration Demercuration of Alkenes
- Hydroboration Oxidation of Alkenes
- m-CPBA (meta-chloroperoxybenzoic acid)
- OsO4 (Osmium Tetroxide) for Dihydroxylation of Alkenes
- Palladium on Carbon (Pd/C) for Catalytic Hydrogenation of Alkenes
- Cyclopropanation of Alkenes
- A Fourth Alkene Addition Pattern - Free Radical Addition
- Alkene Reactions: Ozonolysis
- Summary: Three Key Families Of Alkene Reaction Mechanisms
- Synthesis (4) - Alkene Reaction Map, Including Alkyl Halide Reactions
- Alkene Reactions Practice Problems
13 Alkyne Reactions
- Acetylides from Alkynes, And Substitution Reactions of Acetylides
- Partial Reduction of Alkynes With Lindlar's Catalyst
- Partial Reduction of Alkynes With Na/NH3 To Obtain Trans Alkenes
- Alkyne Hydroboration With "R2BH"
- Hydration and Oxymercuration of Alkynes
- Hydrohalogenation of Alkynes
- Alkyne Halogenation: Bromination, Chlorination, and Iodination of Alkynes
- Alkyne Reactions - The "Concerted" Pathway
- Alkenes To Alkynes Via Halogenation And Elimination Reactions
- Alkynes Are A Blank Canvas
- Synthesis (5) - Reactions of Alkynes
- Alkyne Reactions Practice Problems With Answers
14 Alcohols, Epoxides and Ethers
- Alcohols - Nomenclature and Properties
- Alcohols Can Act As Acids Or Bases (And Why It Matters)
- Alcohols - Acidity and Basicity
- The Williamson Ether Synthesis
- Ethers From Alkenes, Tertiary Alkyl Halides and Alkoxymercuration
- Alcohols To Ethers via Acid Catalysis
- Cleavage Of Ethers With Acid
- Epoxides - The Outlier Of The Ether Family
- Opening of Epoxides With Acid
- Epoxide Ring Opening With Base
- Making Alkyl Halides From Alcohols
- Tosylates And Mesylates
- PBr3 and SOCl2
- Elimination Reactions of Alcohols
- Elimination of Alcohols To Alkenes With POCl3
- Alcohol Oxidation: "Strong" and "Weak" Oxidants
- Demystifying The Mechanisms of Alcohol Oxidations
- Protecting Groups For Alcohols
- Thiols And Thioethers
- Calculating the oxidation state of a carbon
- Oxidation and Reduction in Organic Chemistry
- Oxidation Ladders
- SOCl2 Mechanism For Alcohols To Alkyl Halides: SN2 versus SNi
- Alcohol Reactions Roadmap (PDF)
- Alcohol Reaction Practice Problems
- Epoxide Reaction Quizzes
- Oxidation and Reduction Practice Quizzes
15 Organometallics
- What's An Organometallic?
- Formation of Grignard and Organolithium Reagents
- Organometallics Are Strong Bases
- Reactions of Grignard Reagents
- Protecting Groups In Grignard Reactions
- Synthesis Problems Involving Grignard Reagents
- Grignard Reactions And Synthesis (2)
- Organocuprates (Gilman Reagents): How They're Made
- Gilman Reagents (Organocuprates): What They're Used For
- The Heck, Suzuki, and Olefin Metathesis Reactions (And Why They Don't Belong In Most Introductory Organic Chemistry Courses)
- Reaction Map: Reactions of Organometallics
- Grignard Practice Problems
16 Spectroscopy
- Degrees of Unsaturation (or IHD, Index of Hydrogen Deficiency)
- Conjugation And Color (+ How Bleach Works)
- Introduction To UV-Vis Spectroscopy
- UV-Vis Spectroscopy: Absorbance of Carbonyls
- UV-Vis Spectroscopy: Practice Questions
- Bond Vibrations, Infrared Spectroscopy, and the "Ball and Spring" Model
- Infrared Spectroscopy: A Quick Primer On Interpreting Spectra
- IR Spectroscopy: 4 Practice Problems
- 1H NMR: How Many Signals?
- Homotopic, Enantiotopic, Diastereotopic
- Diastereotopic Protons in 1H NMR Spectroscopy: Examples
- 13-C NMR - How Many Signals
- Liquid Gold: Pheromones In Doe Urine
- Natural Product Isolation (1) - Extraction
- Natural Product Isolation (2) - Purification Techniques, An Overview
- Structure Determination Case Study: Deer Tarsal Gland Pheromone
17 Dienes and MO Theory
- What To Expect In Organic Chemistry 2
- Are these molecules conjugated?
- Conjugation And Resonance In Organic Chemistry
- Bonding And Antibonding Pi Orbitals
- Molecular Orbitals of The Allyl Cation, Allyl Radical, and Allyl Anion
- Pi Molecular Orbitals of Butadiene
- Reactions of Dienes: 1,2 and 1,4 Addition
- Thermodynamic and Kinetic Products
- More On 1,2 and 1,4 Additions To Dienes
- s-cis and s-trans
- The Diels-Alder Reaction
- Cyclic Dienes and Dienophiles in the Diels-Alder Reaction
- Stereochemistry of the Diels-Alder Reaction
- Exo vs Endo Products In The Diels Alder: How To Tell Them Apart
- HOMO and LUMO In the Diels Alder Reaction
- Why Are Endo vs Exo Products Favored in the Diels-Alder Reaction?
- Diels-Alder Reaction: Kinetic and Thermodynamic Control
- The Retro Diels-Alder Reaction
- The Intramolecular Diels Alder Reaction
- Regiochemistry In The Diels-Alder Reaction
- The Cope and Claisen Rearrangements
- Electrocyclic Reactions
- Electrocyclic Ring Opening And Closure (2) - Six (or Eight) Pi Electrons
- Diels Alder Practice Problems
- Molecular Orbital Theory Practice
18 Aromaticity
- Introduction To Aromaticity
- Rules For Aromaticity
- Huckel's Rule: What Does 4n+2 Mean?
- Aromatic, Non-Aromatic, or Antiaromatic? Some Practice Problems
- Antiaromatic Compounds and Antiaromaticity
- The Pi Molecular Orbitals of Benzene
- The Pi Molecular Orbitals of Cyclobutadiene
- Frost Circles
- Aromaticity Practice Quizzes
19 Reactions of Aromatic Molecules
- Electrophilic Aromatic Substitution: Introduction
- Activating and Deactivating Groups In Electrophilic Aromatic Substitution
- Electrophilic Aromatic Substitution - The Mechanism
- Ortho-, Para- and Meta- Directors in Electrophilic Aromatic Substitution
- Understanding Ortho, Para, and Meta Directors
- Why are halogens ortho- para- directors?
- Disubstituted Benzenes: The Strongest Electron-Donor "Wins"
- Electrophilic Aromatic Substitutions (1) - Halogenation of Benzene
- Electrophilic Aromatic Substitutions (2) - Nitration and Sulfonation
- EAS Reactions (3) - Friedel-Crafts Acylation and Friedel-Crafts Alkylation
- Intramolecular Friedel-Crafts Reactions
- Nucleophilic Aromatic Substitution (NAS)
- Nucleophilic Aromatic Substitution (2) - The Benzyne Mechanism
- Reactions on the "Benzylic" Carbon: Bromination And Oxidation
- The Wolff-Kishner, Clemmensen, And Other Carbonyl Reductions
- More Reactions on the Aromatic Sidechain: Reduction of Nitro Groups and the Baeyer Villiger
- Aromatic Synthesis (1) - "Order Of Operations"
- Synthesis of Benzene Derivatives (2) - Polarity Reversal
- Aromatic Synthesis (3) - Sulfonyl Blocking Groups
- Birch Reduction
- Synthesis (7): Reaction Map of Benzene and Related Aromatic Compounds
- Aromatic Reactions and Synthesis Practice
- Electrophilic Aromatic Substitution Practice Problems
20 Aldehydes and Ketones
- What's The Alpha Carbon In Carbonyl Compounds?
- Nucleophilic Addition To Carbonyls
- Aldehydes and Ketones: 14 Reactions With The Same Mechanism
- Sodium Borohydride (NaBH4) Reduction of Aldehydes and Ketones
- Grignard Reagents For Addition To Aldehydes and Ketones
- Wittig Reaction
- Hydrates, Hemiacetals, and Acetals
- Imines - Properties, Formation, Reactions, and Mechanisms
- All About Enamines
- Breaking Down Carbonyl Reaction Mechanisms: Reactions of Anionic Nucleophiles (Part 2)
- Aldehydes Ketones Reaction Practice
21 Carboxylic Acid Derivatives
- Nucleophilic Acyl Substitution (With Negatively Charged Nucleophiles)
- Addition-Elimination Mechanisms With Neutral Nucleophiles (Including Acid Catalysis)
- Basic Hydrolysis of Esters - Saponification
- Transesterification
- Proton Transfer
- Fischer Esterification - Carboxylic Acid to Ester Under Acidic Conditions
- Lithium Aluminum Hydride (LiAlH4) For Reduction of Carboxylic Acid Derivatives
- LiAlH[Ot-Bu]3 For The Reduction of Acid Halides To Aldehydes
- Di-isobutyl Aluminum Hydride (DIBAL) For The Partial Reduction of Esters and Nitriles
- Amide Hydrolysis
- Thionyl Chloride (SOCl2)
- Diazomethane (CH2N2)
- Carbonyl Chemistry: Learn Six Mechanisms For the Price Of One
- Making Music With Mechanisms (PADPED)
- Carboxylic Acid Derivatives Practice Questions
22 Enols and Enolates
- Keto-Enol Tautomerism
- Enolates - Formation, Stability, and Simple Reactions
- Kinetic Versus Thermodynamic Enolates
- Aldol Addition and Condensation Reactions
- Reactions of Enols - Acid-Catalyzed Aldol, Halogenation, and Mannich Reactions
- Claisen Condensation and Dieckmann Condensation
- Decarboxylation
- The Malonic Ester and Acetoacetic Ester Synthesis
- The Michael Addition Reaction and Conjugate Addition
- The Robinson Annulation
- Haloform Reaction
- The Hell–Volhard–Zelinsky Reaction
- Enols and Enolates Practice Quizzes
23 Amines
- The Amide Functional Group: Properties, Synthesis, and Nomenclature
- Basicity of Amines And pKaH
- 5 Key Basicity Trends of Amines
- The Mesomeric Effect And Aromatic Amines
- Nucleophilicity of Amines
- Alkylation of Amines (Sucks!)
- Reductive Amination
- The Gabriel Synthesis
- Some Reactions of Azides
- The Hofmann Elimination
- The Hofmann and Curtius Rearrangements
- The Cope Elimination
- Protecting Groups for Amines - Carbamates
- The Strecker Synthesis of Amino Acids
- Introduction to Peptide Synthesis
- Reactions of Diazonium Salts: Sandmeyer and Related Reactions
- Amine Practice Questions
24 Carbohydrates
- D and L Notation For Sugars
- Pyranoses and Furanoses: Ring-Chain Tautomerism In Sugars
- What is Mutarotation?
- Reducing Sugars
- The Big Damn Post Of Carbohydrate-Related Chemistry Definitions
- The Haworth Projection
- Converting a Fischer Projection To A Haworth (And Vice Versa)
- Reactions of Sugars: Glycosylation and Protection
- The Ruff Degradation and Kiliani-Fischer Synthesis
- Isoelectric Points of Amino Acids (and How To Calculate Them)
- Carbohydrates Practice
- Amino Acid Quizzes
25 Fun and Miscellaneous
- A Gallery of Some Interesting Molecules From Nature
- Screw Organic Chemistry, I'm Just Going To Write About Cats
- On Cats, Part 1: Conformations and Configurations
- On Cats, Part 2: Cat Line Diagrams
- On Cats, Part 4: Enantiocats
- On Cats, Part 6: Stereocenters
- Organic Chemistry Is Shit
- The Organic Chemistry Behind "The Pill"
- Maybe they should call them, "Formal Wins" ?
- Why Do Organic Chemists Use Kilocalories?
- The Principle of Least Effort
- Organic Chemistry GIFS - Resonance Forms
- Reproducibility In Organic Chemistry
- What Holds The Nucleus Together?
- How Reactions Are Like Music
- Organic Chemistry and the New MCAT
26 Organic Chemistry Tips and Tricks
- Common Mistakes: Formal Charges Can Mislead
- Partial Charges Give Clues About Electron Flow
- Draw The Ugly Version First
- Organic Chemistry Study Tips: Learn the Trends
- The 8 Types of Arrows In Organic Chemistry, Explained
- Top 10 Skills To Master Before An Organic Chemistry 2 Final
- Common Mistakes with Carbonyls: Carboxylic Acids... Are Acids!
- Planning Organic Synthesis With "Reaction Maps"
- Alkene Addition Pattern #1: The "Carbocation Pathway"
- Alkene Addition Pattern #2: The "Three-Membered Ring" Pathway
- Alkene Addition Pattern #3: The "Concerted" Pathway
- Number Your Carbons!
- The 4 Major Classes of Reactions in Org 1
- How (and why) electrons flow
- Grossman's Rule
- Three Exam Tips
- A 3-Step Method For Thinking Through Synthesis Problems
- Putting It Together
- Putting Diels-Alder Products in Perspective
- The Ups and Downs of Cyclohexanes
- The Most Annoying Exceptions in Org 1 (Part 1)
- The Most Annoying Exceptions in Org 1 (Part 2)
- The Marriage May Be Bad, But the Divorce Still Costs Money
- 9 Nomenclature Conventions To Know
- Nucleophile attacks Electrophile
27 Case Studies of Successful O-Chem Students
- Success Stories: How Corina Got The The "Hard" Professor - And Got An A+ Anyway
- How Helena Aced Organic Chemistry
- From a "Drop" To B+ in Org 2 – How A Hard Working Student Turned It Around
- How Serge Aced Organic Chemistry
- Success Stories: How Zach Aced Organic Chemistry 1
- Success Stories: How Kari Went From C– to B+
- How Esther Bounced Back From a "C" To Get A's In Organic Chemistry 1 And 2
- How Tyrell Got The Highest Grade In Her Organic Chemistry Course
- This Is Why Students Use Flashcards
- Success Stories: How Stu Aced Organic Chemistry
- How John Pulled Up His Organic Chemistry Exam Grades
- Success Stories: How Nathan Aced Organic Chemistry (Without It Taking Over His Life)
- How Chris Aced Org 1 and Org 2
- Interview: How Jay Got an A+ In Organic Chemistry
- How to Do Well in Organic Chemistry: One Student's Advice
- "America's Top TA" Shares His Secrets For Teaching O-Chem
- "Organic Chemistry Is Like..." - A Few Metaphors
- How To Do Well In Organic Chemistry: Advice From A Tutor
- Guest post: "I went from being afraid of tests to actually looking forward to them".
Hello thanks for the information…using curly curves to show the mechanism of electrophyllic substitution of benzene,show how the products of the following processes are formed: 1: chlorination
2: nitration
3: friedel craft alkylation
hello, thank you first! i have a question, in the example of methyl benzene, why did the reaction give the product where nitro group is in ortho position in a greater yield than the one where it is in para position?
There are two ortho positions and only one para position. So if it were completely random (and there were no meta) the yield would be 66%. The fact that the yield is 57% shows that the methyl group has some steric influence. The effect becomes much greater as the alkyl group is increased in size.
Have you seen the section on EAS from “Modern Physical Organic Chemistry” (p. 608) by Anslyn? The description is quite clear. In most cases the reaction will be second-order overall, but when there is a bulky group (e.g. t-butyl) the rate of the reverse reaction can be significant. In those cases the reverse reaction starts to have about the same barrier as the rate of deprotonation, which leads to secondary isotope effects.
Hey! Great post however a few questions come to my mind from the texts I have. They have been great and clear but how primary isotopic effects and secondary isotopic effects are affected by the bulkiness of the electrophile have not been described very clearly, and other texts don’t seem have any information of them.
It mentions that primary isotopic effect is seen when the loss of an alpha-hydrogen happens in the slower than the formation of the Wheland-complex. It says this is only the case when the electrophile is a significantly bulkier group.
I’d also like to know how charge-transfer complexes are formed from pi-complexes, but that is a little off-topic and at the risk of digressing I wont talk too much about them (I dont understand them well enough anyway)
Assalam o alaikum.very good explanation sir.i enjoyed a lot reading it because it is giving me strong concepts.much helpful!!
I appreciate sir.
The energy of second intermiediate is more than first in the given energy diagram? ….
There is only one intermediate – the carbocation. Are you referring to the two transition states?
Very nice information about of nitration reaction……👌👌👌👌
OK, glad you found it useful Ajit.
Very good post. Very accurate and extremely detailed. I would suggest that you maybe include an example of electrophilic aromatic substitution using acetic anhydride. It’s a very good test question, and (if the school is legit probably tested it many times) because the mechanism is literally the same, you treat it the same as you would for another compound but people have trouble with it.
Thank you Eric! I agree that it’s a common exam question (and a good one). On a related post on the intramolecular Friedel-Crafts reaction I do include an example of that. Good tip!
Can you give me the mechanism of formation of 1,3 di-nitrobenzene?
It would be in no way different from the mechanism for the nitration of benzene.
Superb explanation.
Wonderful! I was able to get the concepts right.