Conformations and Cycloalkanes
Newman Projection of Butane (and Gauche Conformation)
Last updated: November 6th, 2023 |
The Conformational Isomers (and Newman Projections) of Butane
In two previous articles we’ve discussed the conformations of ethane and propane and saw that their staggered conformations were lower in energy than their eclipsed conformations. The barrier to rotation in each (the “torsional strain” of the higher-energy, eclipsed conformation versus the lower-energy eclipsed conformation) was 3.0 kcal/mol in ethane and 3.4 kcal/mol in propane.
So what about the next hydrocarbon up: butane?
In contrast to ethane and propane, which have a single rotational barrier, the situation for butane is a bit more complicated (or as we sometimes say, “more fun”). The highest energy conformation in butane is about 5 kcal/mol above the lowest-energy conformation. But that’s not the only energy maximum; when rotated about the C2-C3 axis.
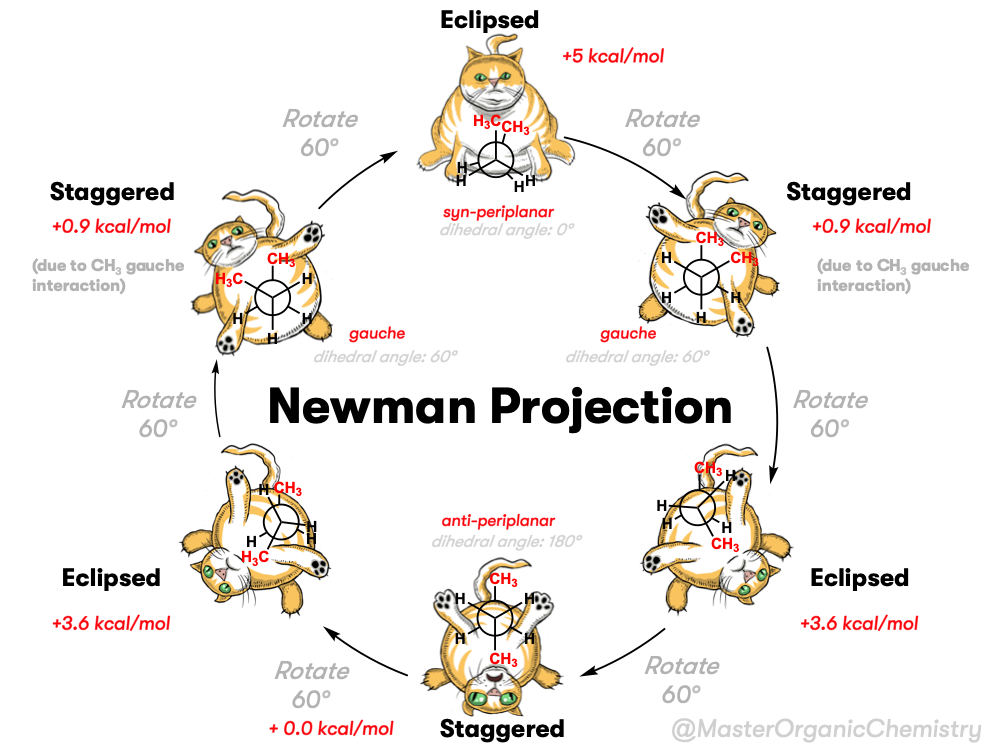
The video below summarizes the full rotational energy profile looks like when we look along the C2-C3 bond and rotate in 60 degree increments.
We can even graph the energy of the different conformations in 60 degree increments.
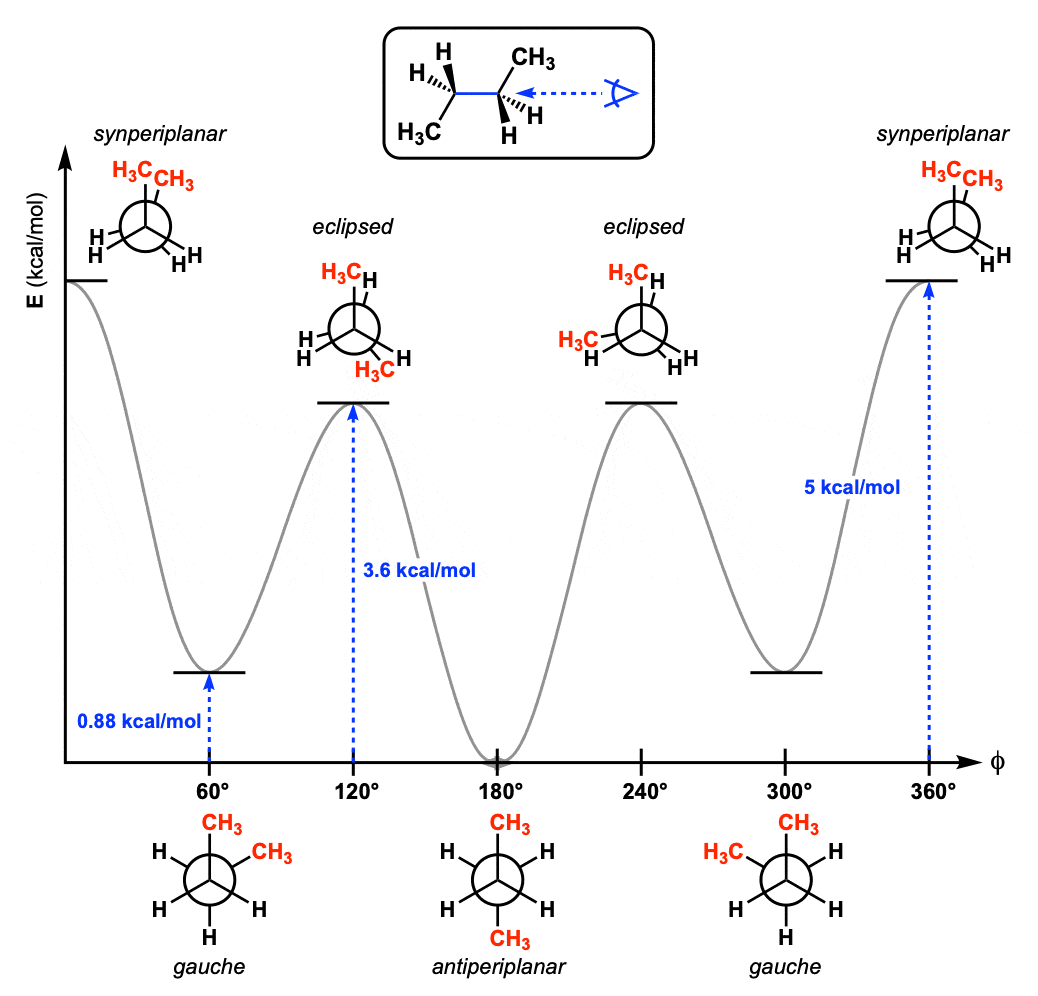
Wait – where did this come from? And what is this “gauche conformation” thing?
Fear not! We’re going to go through how we got here in detail, starting with the absolute basics.
Table of Contents
- Staggered And Eclipsed Conformations Of Butane
- Focus On The Central C2-C3 Bond Of Butane
- Newman Projections Of Butane: Staggered and Eclipsed
- “Steric Interactions” In The syn- Conformation of Butane
- The CH3-CH3 Eclipsing Interaction In Butane “Costs” About 3 kcal/mol
- Two More Eclipsed Conformations Of Butane
- The Gauche Conformation of Butane – Not All Staggered Conformations Are Equal!
- Graphing Out The Conformations of Butane
- Newman Projections of Butane: Conclusion
- Appendix: What About C1-C2?
- Notes
- Quiz Yourself!
- (Advanced) References and Further Reading
1. Staggered And Eclipsed Conformations Of Butane
Let’s get started by drawing butane in the laziest way possible, with all carbons in the plane of the page; the line diagram drawn with the carbons zig-zagging nicely.
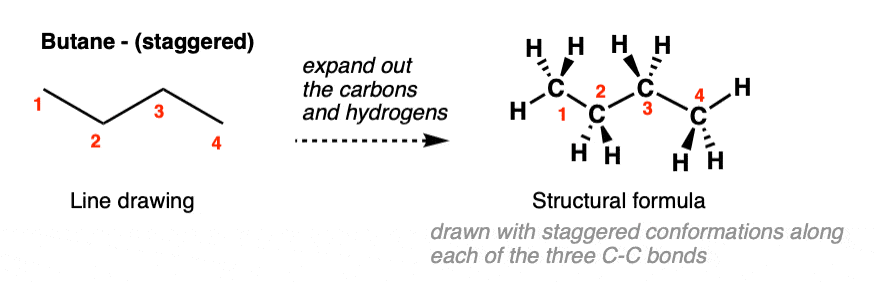
With a bit more effort, we can also expand out the C-H bonds; if we choose to draw all the C-C bonds as staggered, we get the molecule drawn above-right.
Since all the bonds in butane are sigma bonds, and therefore may rotate freely, a perfectly acceptable alternate way to draw butane would be like this, which represents a different conformation of butane where the C2-C3 bond has been notably rotated:
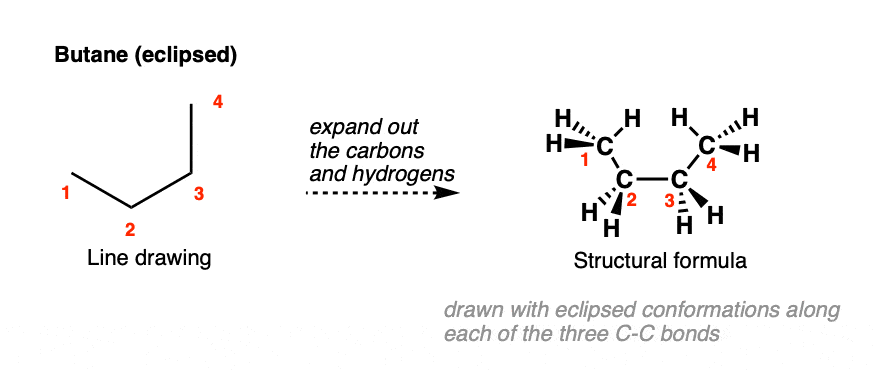
Here, we’ve chosen to draw out the C-H bonds with all the C-C bonds eclipsed.
Since there is free rotation about all the single bonds, these conformations can interconvert.
Model kit time. Here is a gif showing rotation about the C2-C3 bond, interconverting the C2-C3 “staggered” conformation to a C2-C3 “eclipsed” conformation.
So far, so good
[Note 1 – yes, pulled a bit of a fast one there]
2. Focus On The Central C2-C3 Bond Of Butane
Butane has 3 carbon-carbon bonds that can each rotate freely. At first glance, that might seem intimidating to analyze. However, we’re only going to analyze rotation about the central (C2-C3) bond here.
Here’s why.
Say you were at a dinner party, and someone asked you to analyze the whole conformation of butane. Your mind races. “Does the orientation about C1-C2 affect the energy about C2-C3?”.
As it turns out, these three terms are independent and can for our purposes, can be analyzed in isolation. That means that rotations about C1-C2 and C3-C4 don’t affect the torsional strain about C2-C3.
So you’d pull out your pen and calmly do the following analysis:
total torsional strain of butane = [torsional strain of C1-C2 bond] + [torsional strain of C2-C3 bond] + [torsional strain of C3-C4 bond].
This makes our lives much easier, which is why reductionism is awesome.
Also, it turns out that the torsional strain about C1-C2 and C3-C4 in butane is very similar to propane, which we analyzed in the last post.
Bottom line: we’re going to ignore the conformations about C1-C2 and C3-C4 and just focus on the interactions we haven’t seen before.
BTW If you really are aching to see the C1-C2 conformation of butane, it’s in the notes [1]
It will really simplify things from now on if we just draw butane like this:
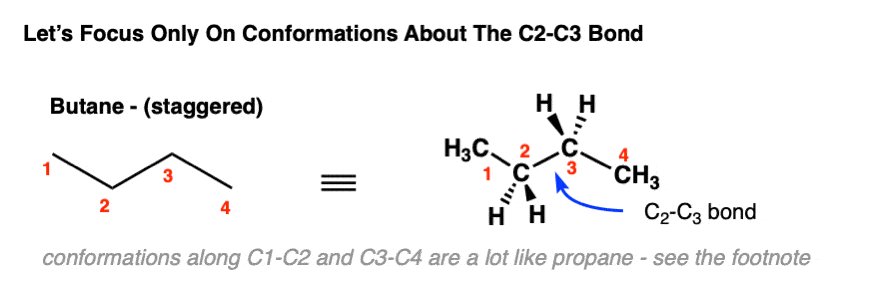
3. Newman Projections Of Butane: Staggered And Eclipsed
Now it’s time for some Newman projections.
Here, we’re going to take our model of butane in the staggered conformation and look along the C2-C3 bond. When we do that we can sketch out the Newman projection like this:
Note that the two methyl groups point up and down like the hour and minute hands on Big Ben when it strikes 6:00 . The dihedral angle between these two methyl groups is 180°; we say that their relative orientation is anti (anti-periplanar to be more specific).
Likewise, if we look along C2-C3 of the eclipsed conformation of butane, we get this:
In this case the two methyl groups have a dihedral angle of 0°, and we say that their relative orientation is syn. [Note 2] [Quick reminder: eclipsed and staggered refer to the orientation of all the substituents on the front with respect to the substituents on the back, while syn and anti refers to relative orientation between two specific groups, although we will muddy the waters sometimes and refer to CH3 – CH3 “eclipsing” interactions and H-H “eclipsing” interactions.]
4. “Steric Interactions” In Butane
Let’s have a closer look at these two conformations. According to these measurements and calculations [Ref 1] the torsional strain of the C2-C3 syn conformation is about 5.0 kcal/mol higher than the C2-C3 anti conformation.
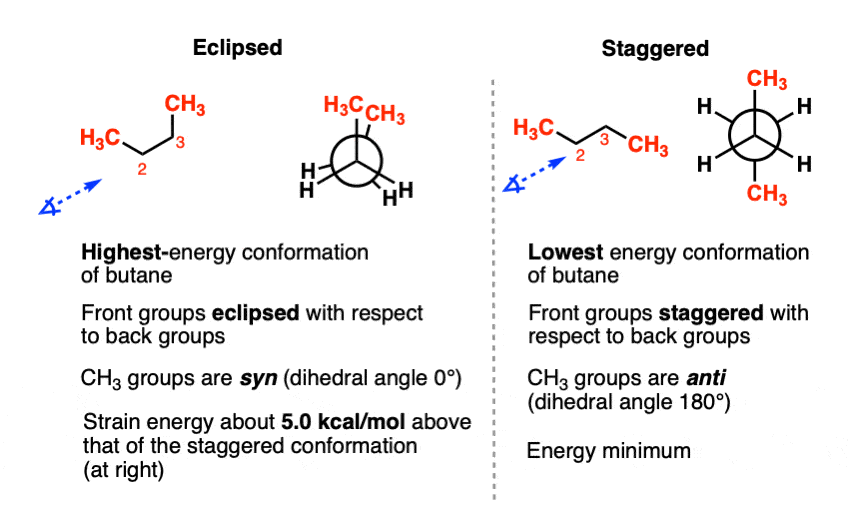
Why so high?
The issue here is that when electron clouds surrounding the hydrogens on the CH3 groups are brought too close together, there are electron-electron repulsions (like charges repel, after all), and this is destabilizing; their natural inclination is to “push away” from each other, much like two magnets of the same polarity push apart when brought close together.
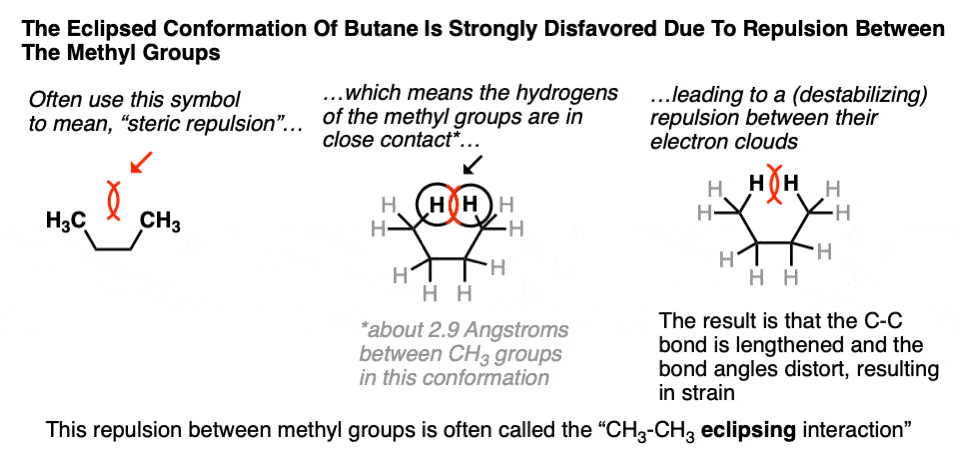
[It’s not unlike repulsions between bonding pairs that give rise to the different molecular geometries of atoms we see in VSEPR theory (trigonal planar, tetrahedral, etc.) , except that these repulsions occur between electrons that are farther away. ]
This interaction in butane is often called the CH3 – CH3 “eclipsing” interaction. It’s an example of what we often call a “steric interaction” or often, colloquially, just “sterics”.
5. The CH3-CH3 Eclipsing Interaction In Butane “Costs” About 3 kcal/mol
If we know that the torsional strain of the CH3-CH3 syn conformation of butane is about 5 kcal/mol relative to CH3-CH3 anti we can figure out how much the CH3-CH3 interaction “costs”.
How so?
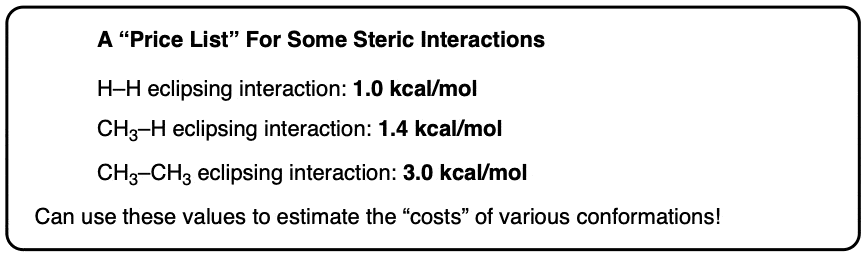
We’ve seen previously that each H-H eclipsing interaction costs about 1 kcal/mol. So if we subtract out the two H-H eclipsing interactions, that leaves us with about 3 kcal/mol for the CH3-CH3 interaction.
If we add the CH3-CH3 eclipsing interaction to our “price list” for steric interactions, we get this.
6. Two More Eclipsed Conformations Of Butane
Now we can start to look at some other conformations of butane. Let’s look at the two other eclipsed conformations, ones where the dihedral angle between the methyl groups is at 120° and 240° respectively.
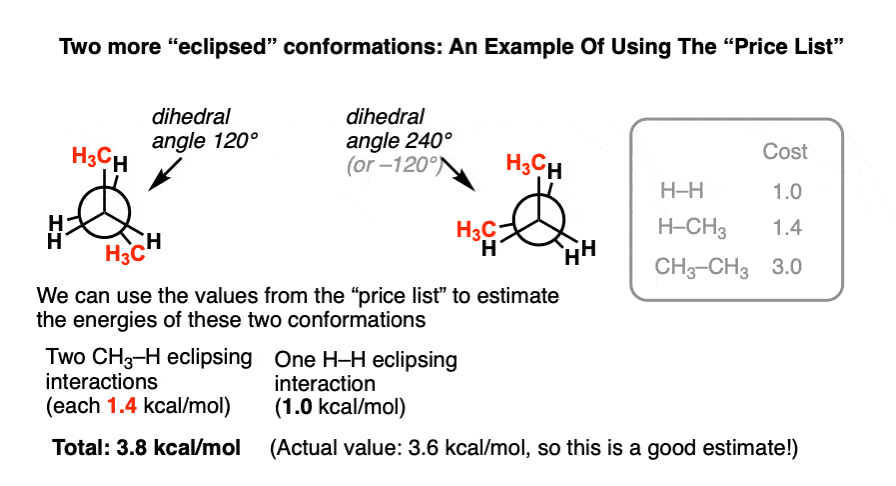
Using the “price list” above, it’s possible to come up with a very good estimate for the torsional strain in this conformation.
We just need to break down all the eclipsing interactions and check their values on the “price list”.
If we add up the two eclipsing CH3-H interactions (1.4 kcal/mol) and the H-H eclipsing interaction (1.0 kcal/mol) we get 3.8 kcal/mol. This is very close to the experimental value of 3.6 kcal/mol.
Reductionism works pretty well!
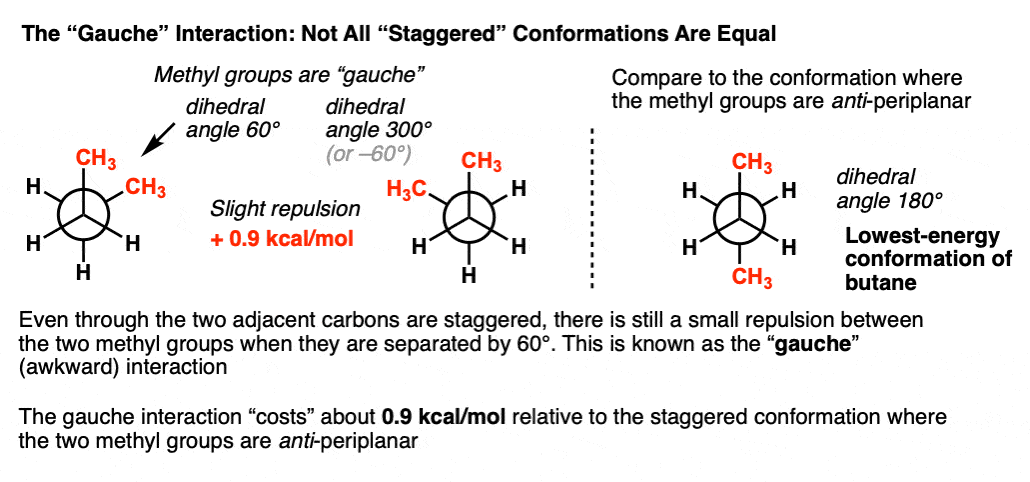
7. Not All Staggered Conformations Are Equal – The Gauche Conformation of Butane
We’re almost done. Let’s look at the two other “staggered” conformations of butane, those with a dihedral angle between methyl groups of 60° and 300° respectively.
Since the substituents on C2 are staggered with respect to the three groups on C3, you might initially think the strain in these conformations is zero. That’s not quite true.
The two conformations have a torsional strain of about +0.9 kcal/mol relative to the anti conformation.
Why? As it turns out these methyl groups are not so far apart. The calculated distance between the two methyl groups is about 3.1 Å, which is not far from the distance of 2.9 Å in the syn orientation, meaning they are still quite close together.
This minor steric interaction has come to be known as the “gauche” conformation – gauche meaning, “awkward”.
The gauche interaction comes up in the chair form of cyclohexane. [Note 3]. A methyl group in the axial position will experience two gauche interactions with the neighboring C-C bonds, resulting in the 1.8 kcal/mol energy difference (or “A-value”) observed for axial vs equatorial methyl groups in cyclohexanes. [See post: Ranking The Bulkiness Of Substituents On Cyclohexanes – “A Values”]
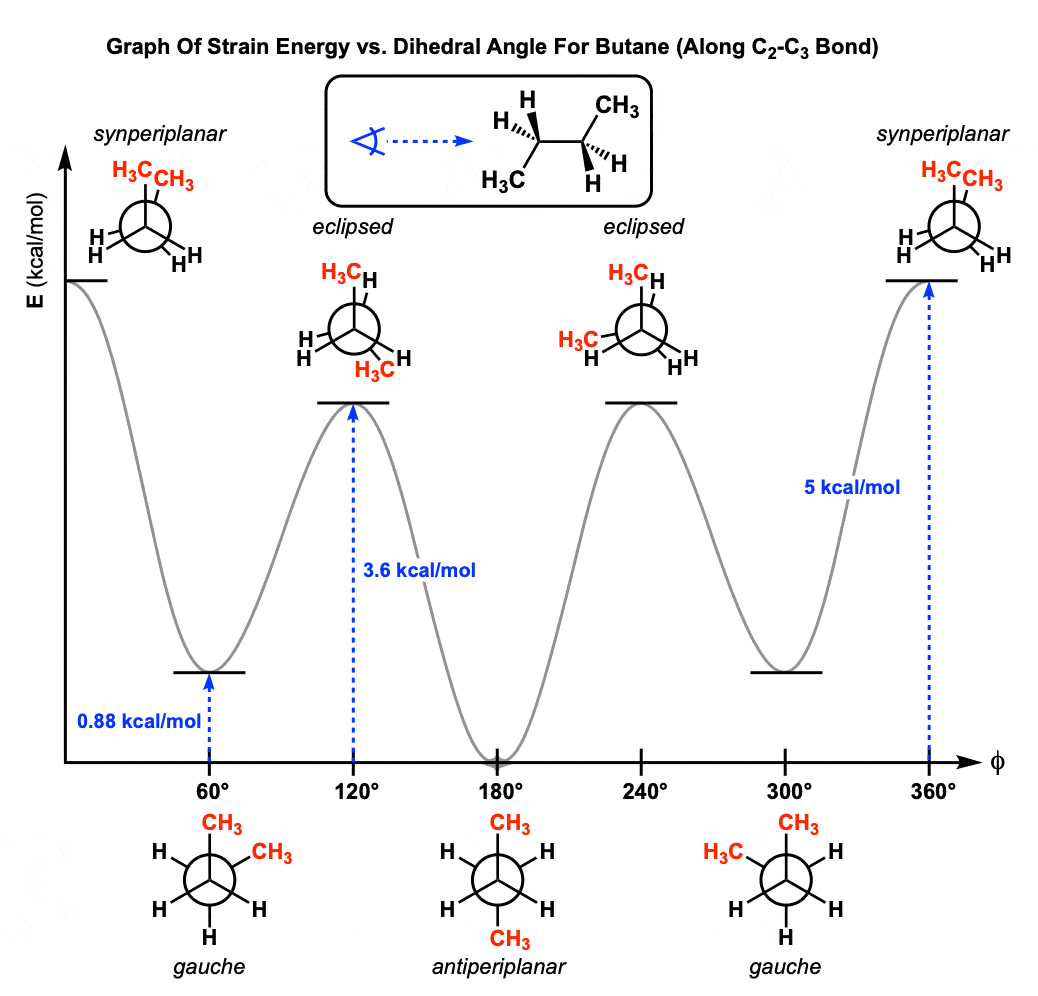
8. Graphing Out The Conformations Of Butane
Now that we’ve explored the conformational isomers of butane (in 60° increments) let’s summarize by showing a Newman projection of butane along the C2-C3 bond with the methyl groups syn and sweep the back methyl group through a rotation of 360°.
We can even graph out each of the conformations of butane and put in a diagram like this. From this graph we can see that butane has three barriers to rotation along the C2-C3 bond.
9. Newman Projections of Butane: Conclusion
So what did we learn? Here are some key points.
- Steric interactions are what we call repulsions between the electron clouds of substituents that come into close contact, such as in the eclipsed conformation of butane where the two methyl groups are syn. In order to reduce steric interactions, bonds and bond angles deform ever-so-slightly from their ideal values, resulting in strain.
- Strain results in small barriers to rotation between conformations, which can be measured experimentally and calculated using advanced computational methods we won’t get into [e.g. Ref 1]
- In contrast to ethane, propane, and the C1-C2 bond of butane, along the C2-C3 bond of butane not all energy maxima (the peaks on the graph above) have the same energy. The energy maxima correspond to the eclipsed conformation with both methyl groups syn (dihedral angle 0°, strain energy about 5 kcal/mol) and the two remaining eclipsed conformations (dihedral angles 120° and 240°, strain energy about 3.6 kcal/mol).
- The C2-C3 bond of butane has two new steric interactions we did not see in ethane or propane. The first is the CH3-CH3 eclipsing interaction, which introduces about 3.0 kcal/mol of strain.
- The gauche interaction occurs in butane occurs when the two methyl groups have dihedral angles of 60° and 300° and arises because the methyl groups are still quite close together (about 3.1 Å, compare to 2.9 Å) for the syn– conformation. The strain energy of the gauche interaction is about 0.9 kcal/mol. (We’ll see this again when we discuss conformations of cyclohexanes.)
- Given a list of the “costs” of various steric interactions, we can make good estimates for the strain energies of various conformations. For the two eclipsed conformations with dihedral angles of 120° and 240° we saw that we could make a pretty good guess of its strain energy using previously determined values of 1 kcal/mol and 1.4 kcal/mol for the H-H and CH3-H eclipsing interactions, respectively . Don’t be surprised to see questions like this on exams! :-)
Appendix – What About The C1 C2 Rotation of Butane?
The C1-C2 bond in butane has a single barrier to rotation, just like propane. The ethyl group isn’t actually that much “bigger” than the methyl group for our purposes, because the CH3 at the end of the methyl group can just point away from C-C, resulting in very little additional strain:
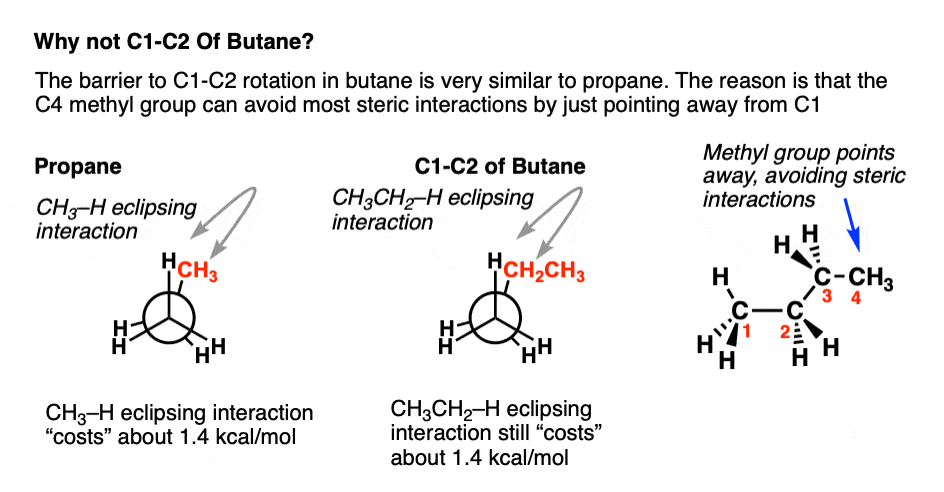
(If you’re familiar with A-values, the A-value of ethyl (1.75 kcal/mol) is only slightly larger than that for methyl (1.7 kcal/mol).
Notes
Many thanks to Jeremy Tran for drawing out the rotational energy profile of butane.
The cat diagram was done by the brilliant Canadian political cartoonist Graeme Mackay who is more used to drawing heads of state.
Note 1. The methyl groups on C1 and C4 were collapsed here as CH3 since otherwise we’d have to show rotation about C1-C2 and C3-C4 to get the “all staggered” conformation to transform to the “all eclipsed” conformation, and that would be annoying.
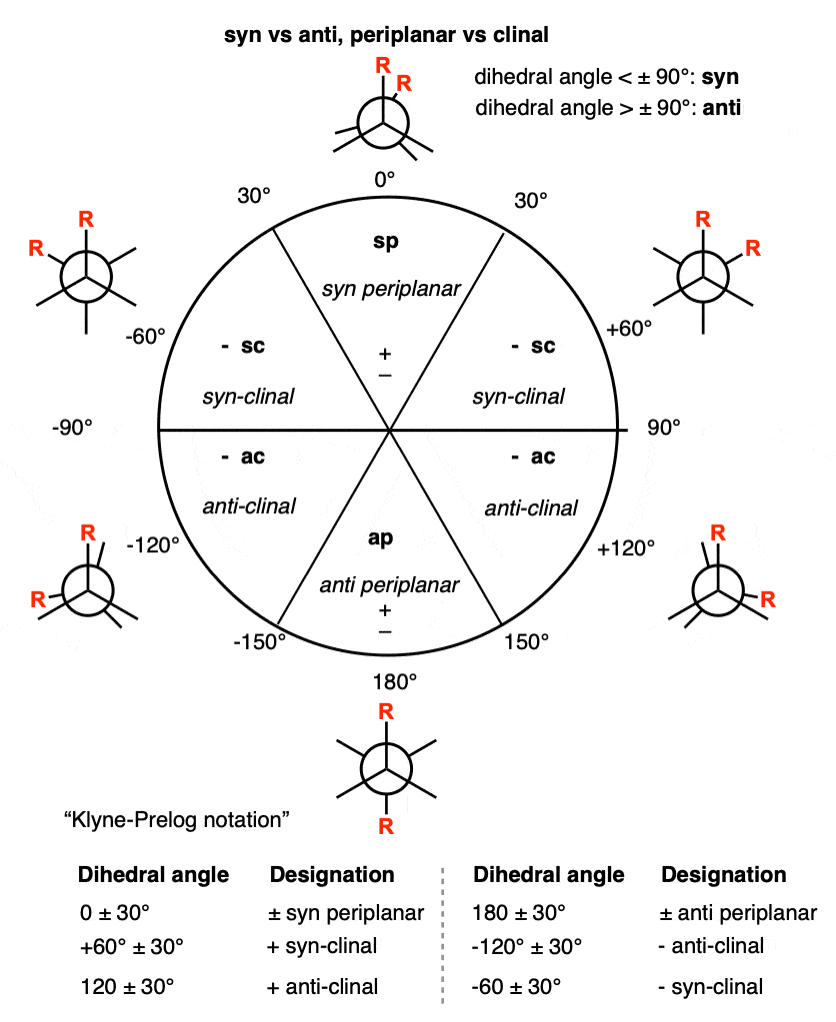
Note 2. If the dihedral angle between two substituents is acute (ranges from -90° < 0 < +90°) they are said to be “syn”. If the dihedral angle is obtuse +90°< 180 <270° (-90°) they are “anti”.
Between the dihedral angles of +30° and -30° and 150° and -150° (210°) two substituents are said to be “periplanar”, i.e. in the same plane (or roughly so). Substituents that are not periplanar are said to be “clinal” (+30° to 150° and -30° to -150°).
So there are four possibilities; syn-periplanar, anti-periplanar, syn-clinal (+ and -) and anti-clinal (+ and -). We generally don’t discuss clinal in introductory organic chemistry (since it interferes with our discussion of gauche) but hey, now you know. The terminology comes from Klyne and Prelog (Ref 6)
Note 3. If you compare the axial and equatorial conformations of 1-methylcyclohexane, the axial conformation has two gauche interactions that the equatorial methyl group does not have. These roughly total 1.7 kcal/mol.
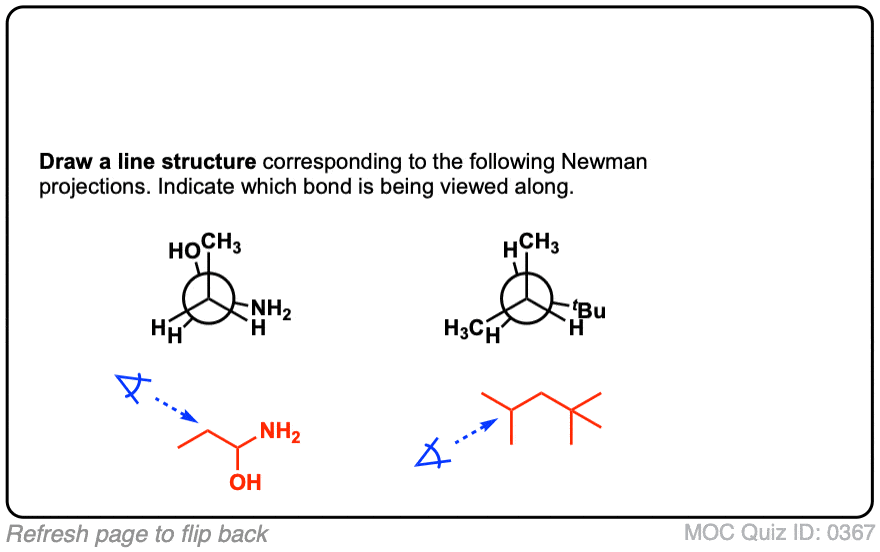
Quiz Yourself!
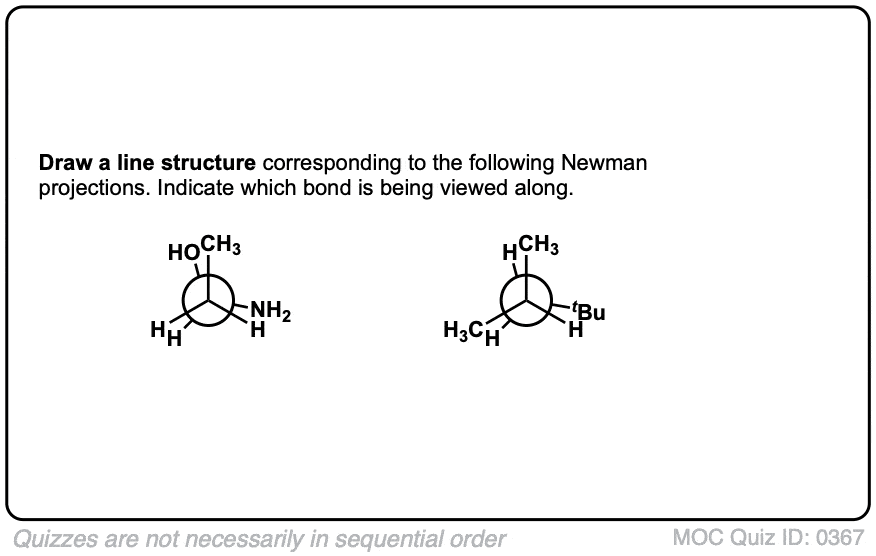 Click to Flip
Click to Flip
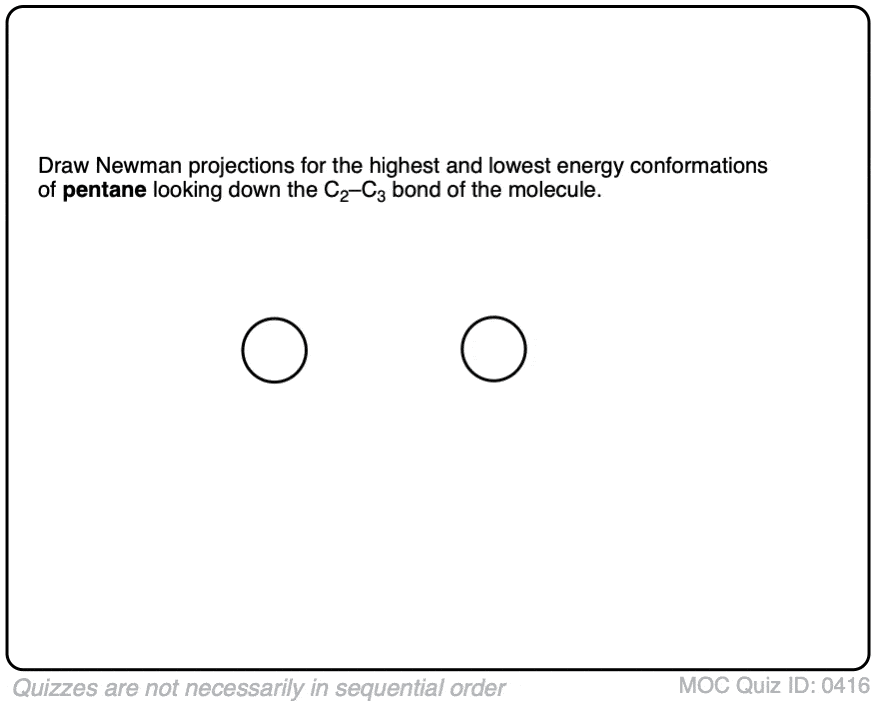 Click to Flip
Click to Flip
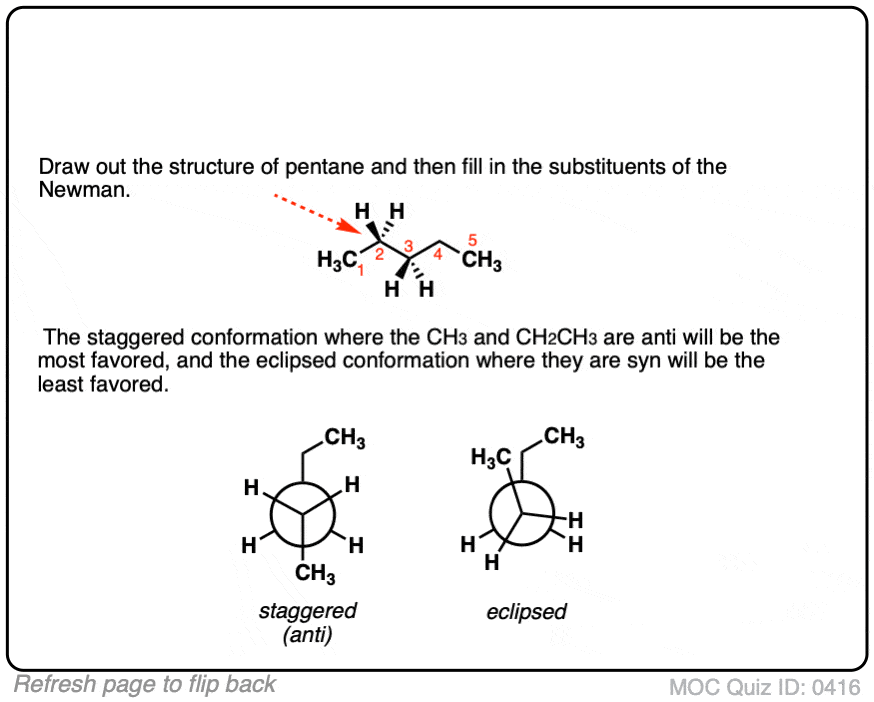
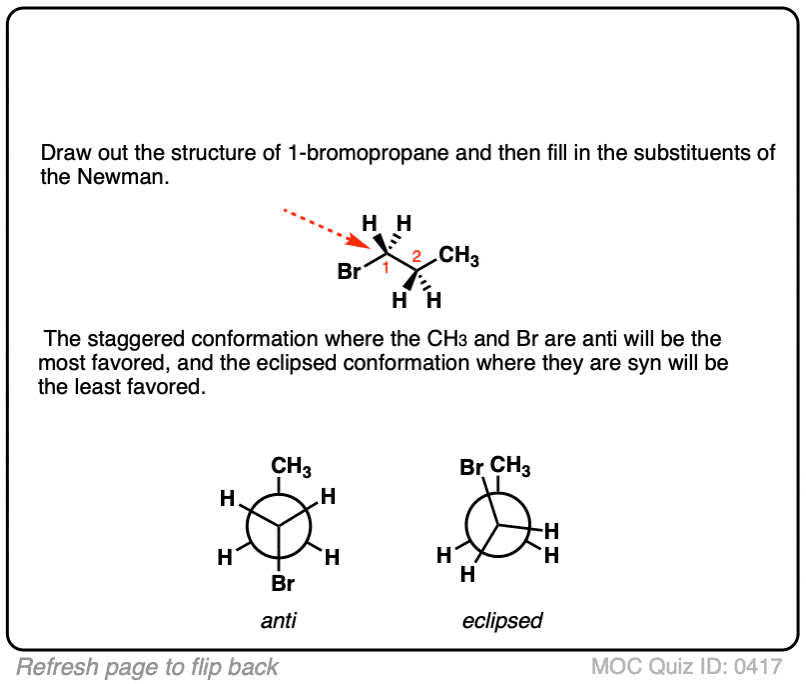
 Click to Flip
Click to Flip
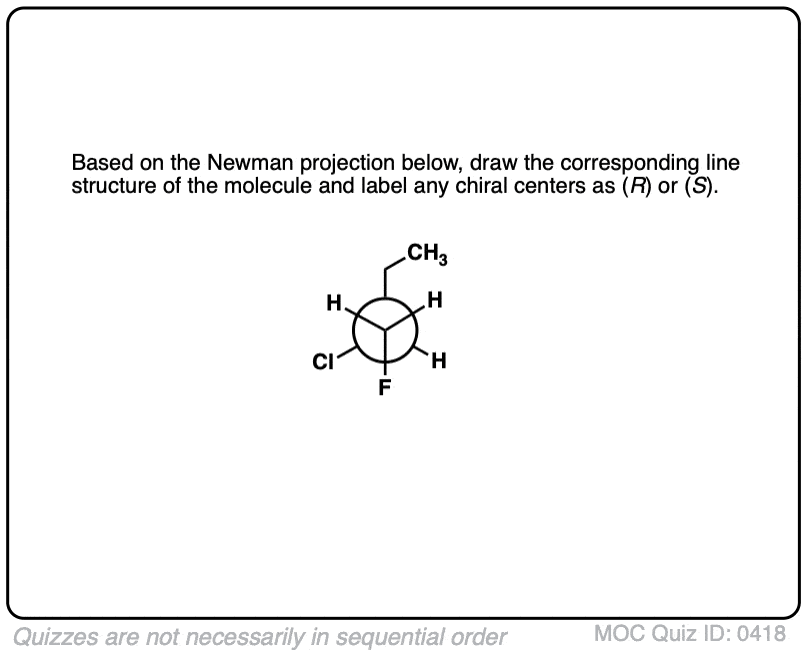 Click to Flip
Click to Flip
(Advanced) References and Further Reading
The value for the rotational barrier of butane has measured by several experimental techniques, as well as calculated by theoretical methods. These methods do not always agree. Eliel’s Stereochemistry of Organic Compounds has a much deeper discussion of these issues than you will find in any other textbook or online resource. Textbooks (and Wikipedia, which cites 19 kJ/mol (4.5 kcal/mol in a diagram and 5.0 kcal/mol in the text) differ in their published values for the rotational barrier for butane.
- The syn rotational barrier in butane
Norman L. Allinger, Roger S. Grev, Brian F. Yates, and Henry F. Schaefer III
Journal of the American Chemical Society 1990, 112 (1), 114-118
DOI: 1021/ja00157a018
This is the source of the values used for the conformational barriers in butane in this article (0.9 kcal/mol for gauche and 5.1 kcal/mol for butane) calculated by molecular mechanics methods. - Far infrared spectrum and conformational potential function of n-butane
Howard D. Stidham, J.R. Durig
Spectrochimica Acta Part A: Molecular Spectroscopy, 1986 Volume 42, Issues 2–3, 105-111
DOI: 10.1016/0584-8539(86)80169-6
Measurement of the rotational barrier in butane by far-IR spectroscopy. “The s–trans to gauche, gauche to gauche, and gauche to s–trans barriers in cm−1 were found to be: 1315 (3.76 kcal/mol), 1090 (3.12 kcal/mol) and 1070 (3.06 kcal/mol), respectively” - Rotational barriers. 2. Energies of alkane rotamers. An examination of gauche interactions
Kenneth B. Wiberg and Mark A. Murcko
Journal of the American Chemical Society 1988, 110 (24), 8029-8038
DOI: 1021/ja00232a012
Calculation of the rotational barrier in butane by ab initio methods; gives a barrier of >6 kcal/mol. - Carbon−Carbon Rotational Barriers in Butane, 1-Butene, and 1,3-Butadiene
Mark A. Murcko, Henry Castejon, and Kenneth B. Wiberg
The Journal of Physical Chemistry 1996, 100 (40), 16162-16168
DOI: 1021/jp9621742
“The difference in energy between the syn and anti forms of butane at 298 K is 5.1 kcal/mol, which is significantly larger than the experimental estimate. However, it is shown that a reliable experimental estimate of the barrier cannot be made based on the currently available data.” - A Critical Analysis on the Rotation Barriers in Butane
Yirong Mo
The Journal of Organic Chemistry 2010, 75 (8), 2733-2736
DOI: 1021/jo1001164
Study on hyperconjugation in the rotational barrier for butane. “[Our] results show that although there are stronger hyperconjugative interactions in the staggered anti and gauche conformers than the eclipsed structures, the energy curve and barriers are dominated by the steric repulsion.” - Description of steric relationships across single bonds.
Klyne, W., Prelog, V.
Experientia 16, 521–523 (1960).
DOI: 10.1007/BF02158433
The Klyne-Prelog notation for stating the conformational isomers of butane.In contrast to ethane and propane, which have a single barrier to rotation, butane has three barriers to rotation, of varying heights, when rotated about the C2-C3 axis.
In the cat drawing by MackayCartoons, shouldn’t the antiperiplaner be 0 kcal/mol and the syn periplaner be 5kcal/mol? I believe they were accidently switched.
Yes, thank you for spotting. They were accidentally switched and this has now been fixed.
Awesome, I am following last couple of months, helpful and improved myself.
Glad you find the site useful.