Reactions of Aromatic Molecules
Reactions on the “Benzylic” Carbon: Bromination And Oxidation
Last updated: March 26th, 2025 |
The carbon adjacent to an aromatic ring – the “benzylic” carbon – can participate in several useful and interesting reactions. Today we’ll provide examples and mechanisms for two key examples: benzylic bromination and benzylic oxidation. Here’s the quick summary:
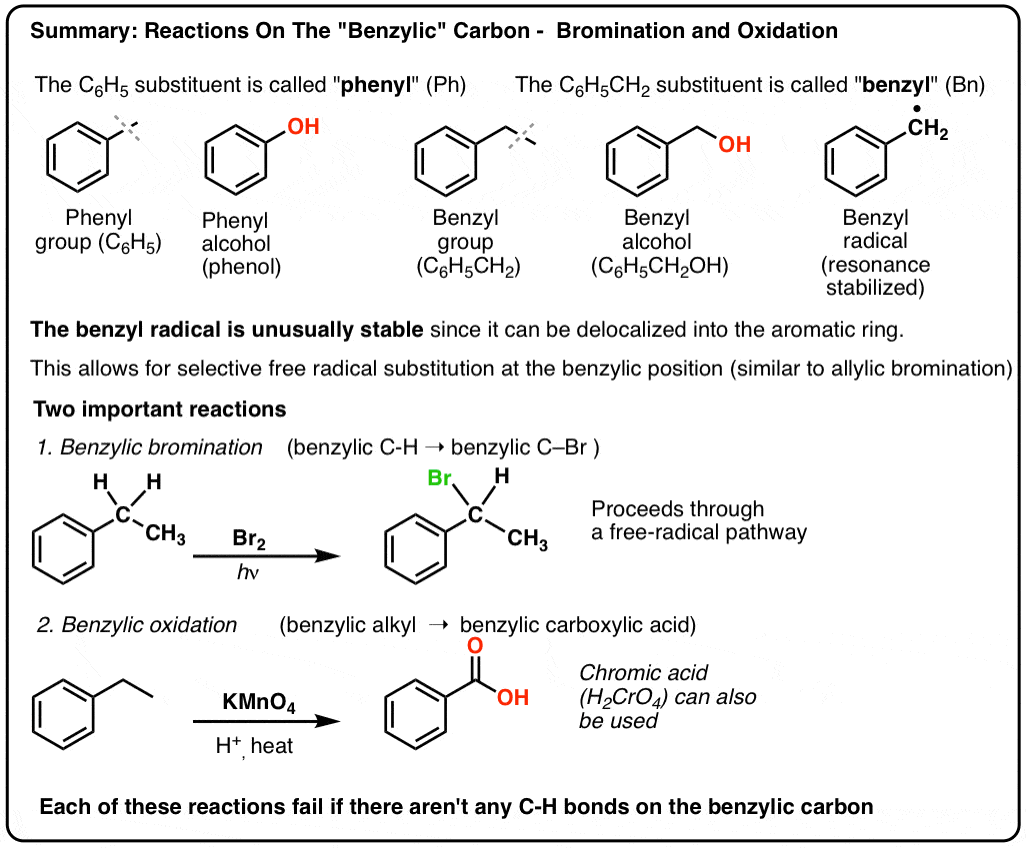
Table of Contents
- Alkyl Groups = “Roughage”
- There’s Something Special About Alkyl Groups Adjacent To A Benzene Ring
- “Phenyl” vs “Benzyl” : What’s The Difference?
- Benzyl Radicals Are Stabilized By Resonance; Phenyl Radicals Are Not.
- Benzylic (and Allylic) C-H Bonds Are Weak, Relative To Other Alkyl C-H Bonds
- Benzylic Bromination
- When Does “Benzylic Bromination” NOT Work?
- Applications of Benzylic Bromination
- Benzylic oxidation With KMnO4 (or H2CrO4)
- Benzylic Oxidation Requires A Benzylic C-H Bond
- Why Is This Important? [Hint: Applications In Synthesis]
- Notes
- Quiz Yourself!
- (Advanced) References and Further Reading
1. Alkyl Groups = “Roughage”
In nutrition, “roughage” is what you call all the cellulose and fiber present in foodstuffs that just shoot through your body without actually being digested.
That pretty much describes the behavior of alkyl groups in the vast majority of the organic chemistry reactions we’ve learned. Their chemistry is “boring”. They may comprise the backbone and overall structure of the molecule, but their C-H bonds are inert to most reaction conditions. [Note 1]
In fact, if you don’t count combustion, pretty much the only reaction of alkyl groups we cover in introductory organic chemistry is free-radical substitution. And even that reaction tends to be pretty unselective, particularly if you’re dealing with free radical chlorination. [See: Selectivity in Free-Radical Reactions]
We’ve seen that an exception to this “boring” behavior can be found in the free-radical bromination of so-called “allylic” C-H bonds [See: Allylic Bromination]. The “allylic” position is a carbon that is adjacent to a double bond (not a C-H on the double bond itself! that’s a “vinylic” C-H).
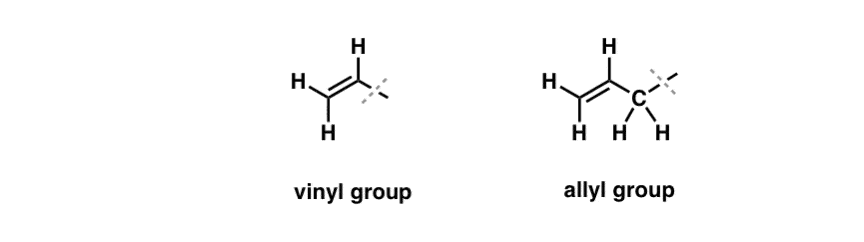
What makes the “allylic” position so special? Allylic radicals, carbocations, and anions are considerably stabilized through resonance. The orbital of the allylic radical, carbocation, or anion can align itself with the p-orbitals in the adjacent pi bond, resulting in a larger “pi system” and spreading out the charge over a greater area. [See: the molecular orbitals of the allyl radical, cation, and anion]
2. There’s Something Special About Alkyl Groups Adjacent To A Benzene Ring
What’s the reason for this little trip down memory lane? Well, we’ve recently spent a little while going over the Friedel-Crafts alkylation reaction, and I don’t want to give you the impression that putting an alkyl group on a benzene ring is a synthetic dead-end.
Alkyl groups don’t have to be inert! Like allylic carbons, alkyl groups that are adjacent to benzene rings are particularly reactive, and this can have all kinds of important applications.
There are two important reactions we will cover in this post:
- Benzylic bromination – free-radical bromination of the alkyl group adjacent to an aromatic ring
- Benzylic oxidation – complete oxidation of an alkyl group adjacent to benzene to a carboxylic acid.
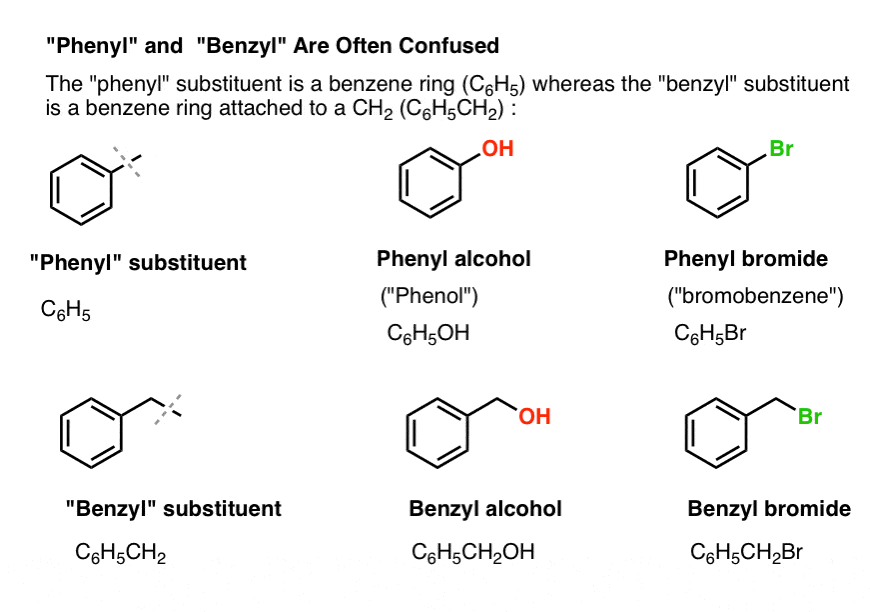
3. “Phenyl” vs “Benzyl” : What’s The Difference?
First, one quick order of business. Let’s get some important nomenclature out of the way, because “phenyl” and “benzyl” cause a lot of confusion:
- A phenyl group (or phenyl substituent) is benzene minus a hydrogen: C6H5 . The carbons in the ring are phenyl carbons, and the hydrogens attached to phenyl carbons are phenyl C-H bonds. It’s analogous to vinyl (see above).
- A benzyl group is methylbenzene minus a hydrogen: C6H5CH2 . The carbon adjacent to the ring is the benzylic carbon, and the hydrogens attached to the benzylic carbon are called benzylic hydrogens. It’s analogous to allyl (above).
4. Benzyl Radicals Are Stabilized By Resonance; Phenyl Radicals Are Not.
The analogy of phenyl with vinyl and benzyl with allyl also extends to the stability of their radicals (as well as their carbocations and anions).
- Phenyl radicals are not stabilized by resonance, since the orbital containing the radical is at right angles to the orbitals of the pi-system, and there is no overlap between them. Radicals on sp2 hybridized carbons are generally less stable than radicals on alkyl carbons. (See post: three factors which destabilize free radicals )
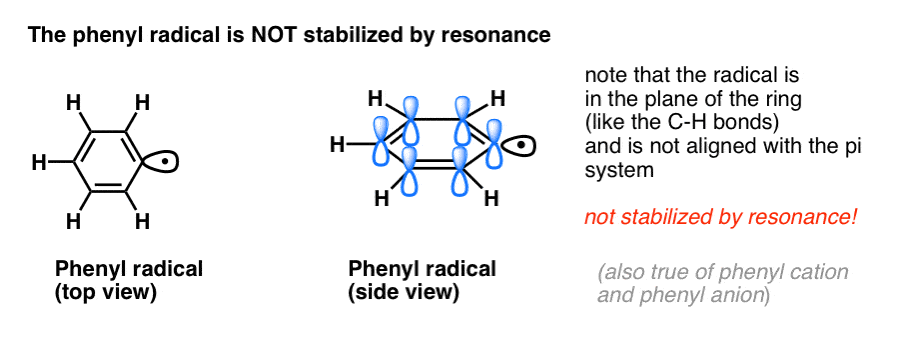
- Benzylic radicals are unusually stable since the orbital containing the radical can align with the pi-system of the benzene ring, just as in their allylic cousins (this is also true for benzyl cations and anions).
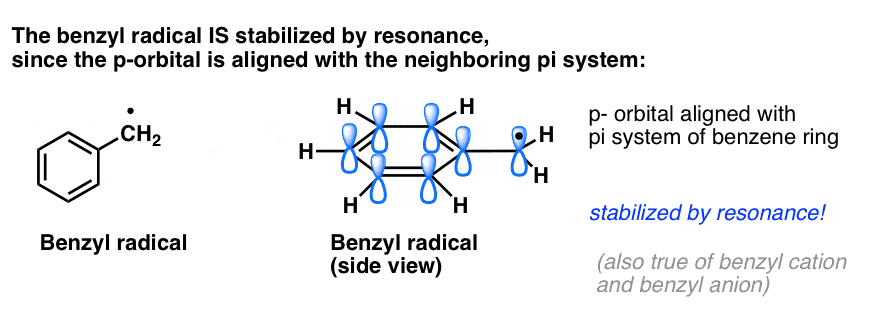
5. Benzylic (and Allylic) C-H Bonds Are Weak, Relative To Other Alkyl C-H Bonds
Benzylic C-H bonds have bond strengths (aka BDE’s, or bond dissociation energies) of about 90 kcal/mol, which is considerably weaker than tertiary C-H (bond strength 93 kcal/mol) secondary C-H (96 kcal/mol) and primary C-H (100 kcal/mol) bonds. (Phenyl C-H bonds are about 113 kcal/mol, again reflecting the instability of phenyl radicals [ref]).
Since bond dissocation energies reflect the ease of homolytic cleavage of the C-H bond (i.e. how easy it is to dissociate the bond into two free radicals) this is another way of saying that benzylic radicals are particularly easy to form, relative to other alkyl radicals.
We’ve previously seen that the same is true for allylic C-H bonds . In ‘allylic bromination’, one of the C-H bonds on the alkyl group adjacent to a double bond is converted to a C-Br bond, using N-bromosuccinimide (NBS) and light (hν) :
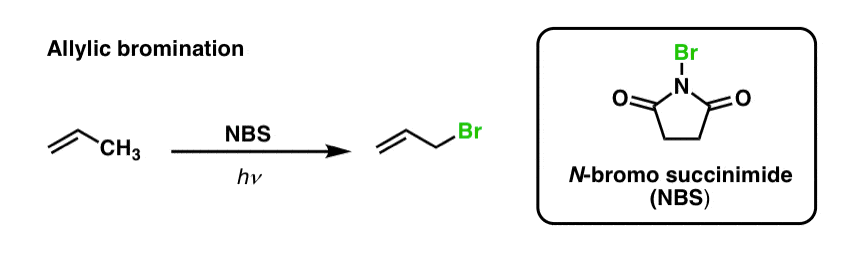
[Why NBS and not Br2 ? The purpose of NBS is to provide a constant, low-level concentration of Br2 that is available for the free-radical substitution reaction. It might seem simpler to just use Br2, but Br2 quickly reacts with double bonds to give vicinal dibromides! ]
The reaction is selective for allylic C-H in the presence of primary, secondary, and tertiary C-H bonds due to the weaker allylic C-H bond strength (89 kcal/mol).
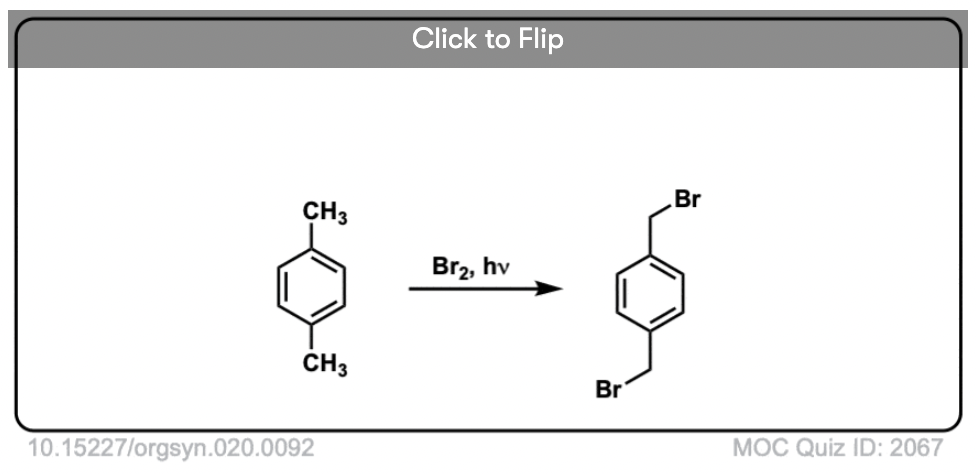
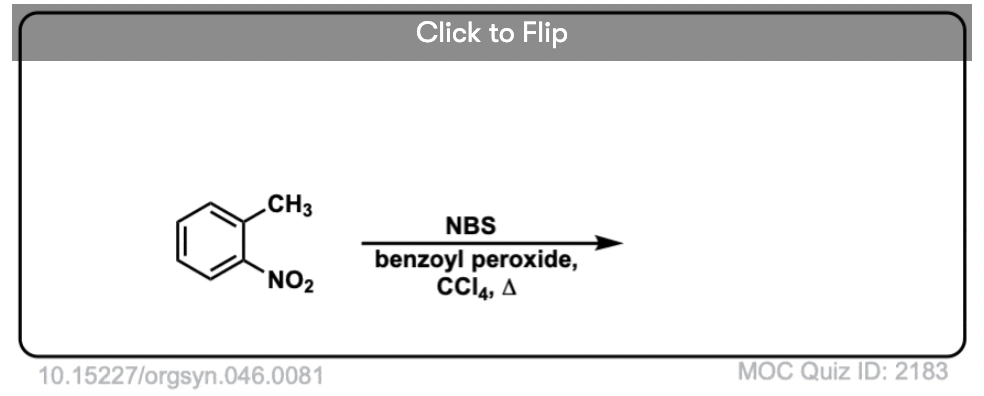
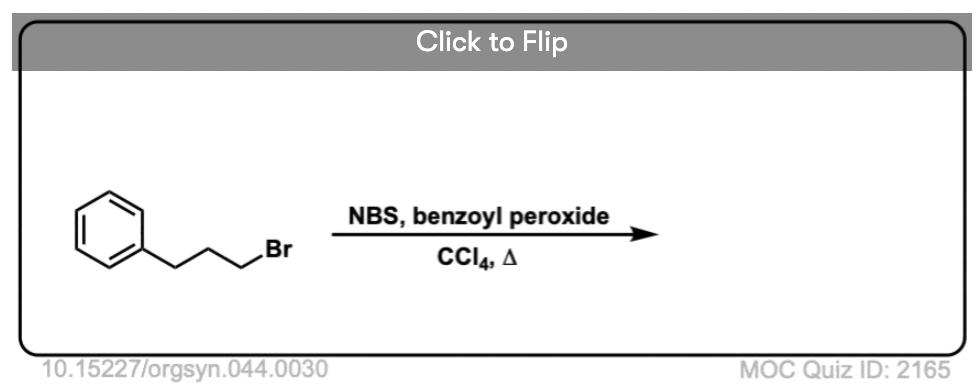
6. Benzylic Bromination
A similar process works for “benzylic” carbons. We can use NBS (as above) or simply just Br2, since aromatic rings aren’t nearly as reactive with Br2 as double bonds are. [Note 2]
Once formed (through “heat” or “light”, which initiates the reaction through homolytic dissociation of Br–Br), the bromine radical, Br• can break the weaker benzylic C-H bonds without touching stronger alkyl C-H bonds.
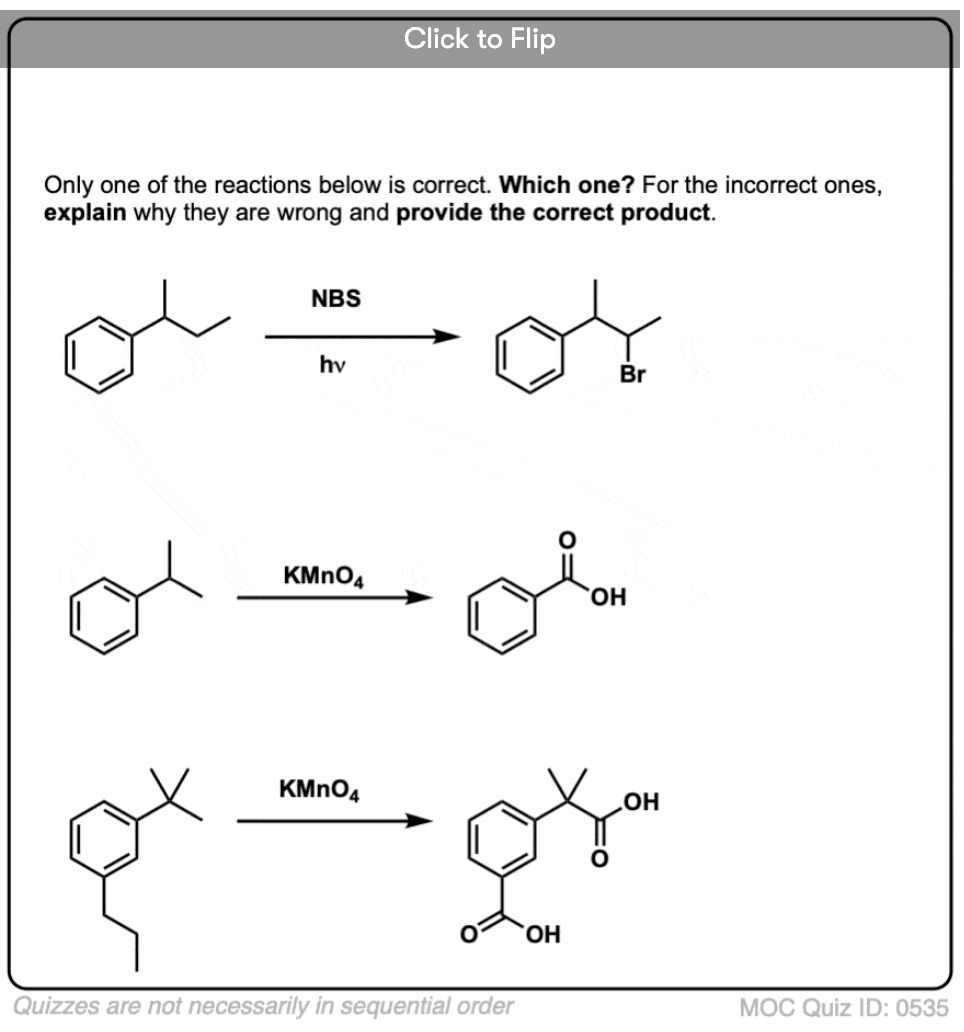
For instance, here are the products of its reaction with toluene, and also isopropylbenzene. Note that ONLY the benzylic carbon is brominated. Everything else is left alone!
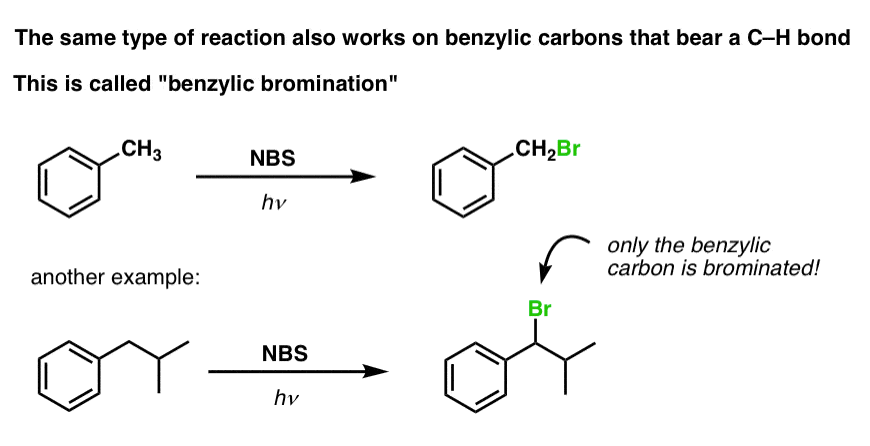
The mechanism of benzylic bromination is essentially identical to allylic bromination, with initiation, propagation, and termination steps.
To see the full mechanism, hover here and an image will pop up or click this link.
(For more discussion, see this post: Allylic Bromination )
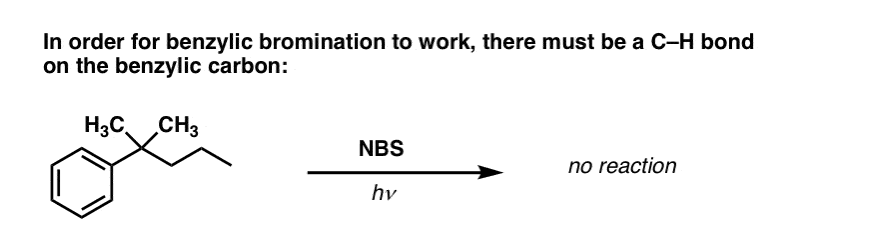
7. When Does “Benzylic Bromination” NOT Work?
Since the key to this reaction is formation of the (relatively stable) benzylic radical, benzylic bromination will fail on substrates where generating this radical via breaking a C–H bond is impossible.
For example, look at the example below. How can a benzylic radical possibly be generated when the benzylic carbon has three alkyl groups attached? It can’t – and the reaction fails.
8. Applications of Benzylic Bromination
So why might benzylic bromination be useful?
Well, it allows us to employ all the reactions of alkyl halides that we covered back in Org 1 – mostly substitution and elimination – to provide products that would be difficult to install directly on an aromatic ring through Friedel-Crafts reactions. [See: Synthesis: Reactions of Alkyl Halides]
For instance, can use benzylic bromination to set up an SN2 reaction, e.g. after a Friedel-Crafts alkylation:
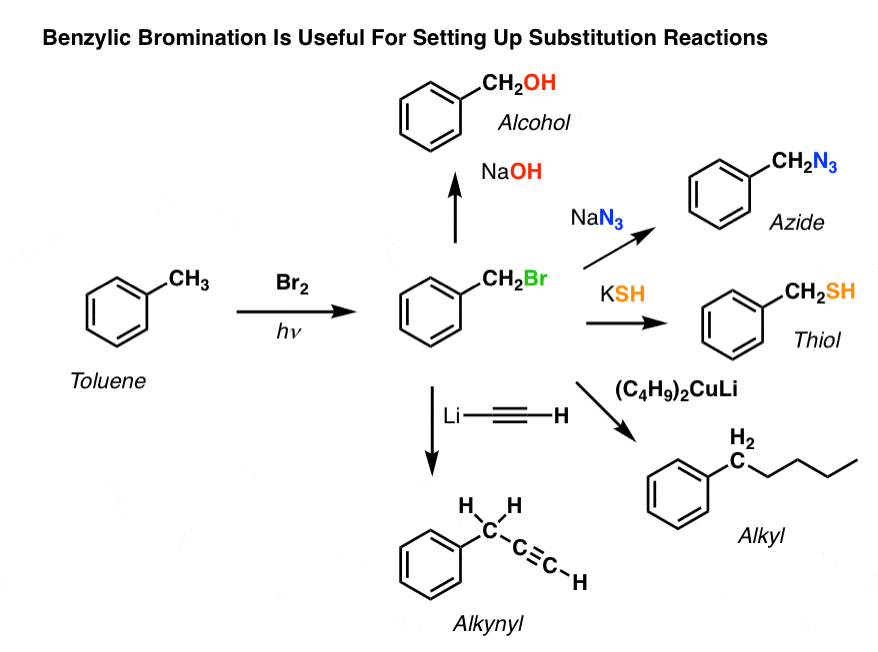
See how installing a good leaving group at the benzylic position gives us all kinds of options for installing various functional groups?
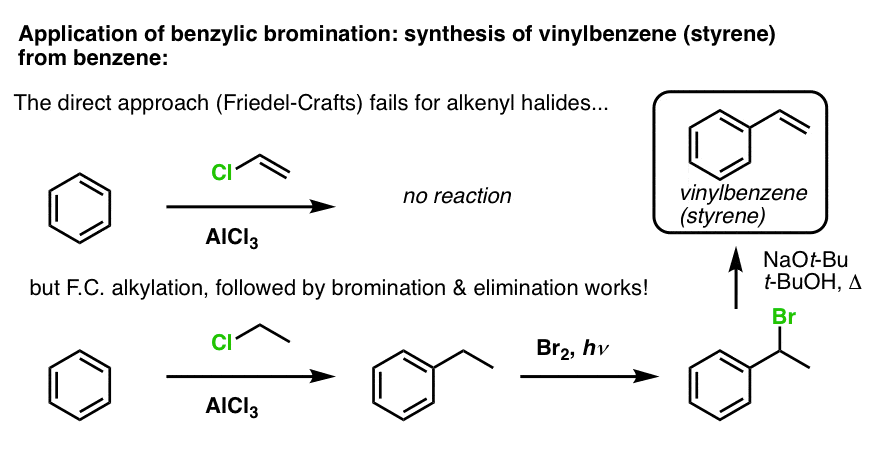
Another example is the synthesis of styrene from benzene. At first glance, the most direct approach might seem to be the Friedel-Crafts reaction of vinyl chloride with benzene. But the Friedel-Crafts doesn’t work for alkenyl halides (aka vinyl halides)! (vinyl carbocations are less stable than alkyl carbocations).
We can get around this roadblock by performing a Friedel-Crafts alkylation with ethyl chloride, followed by benzylic bromination and then elimination (via E2, with something like NaOtBu/t-BuOH) to give the alkene:
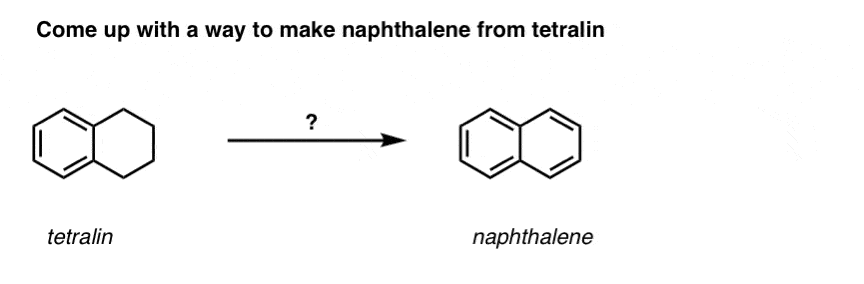
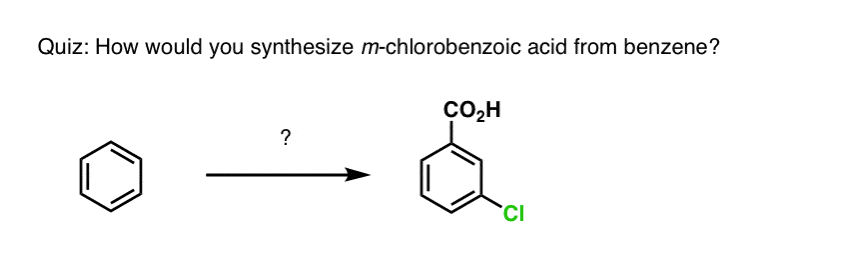
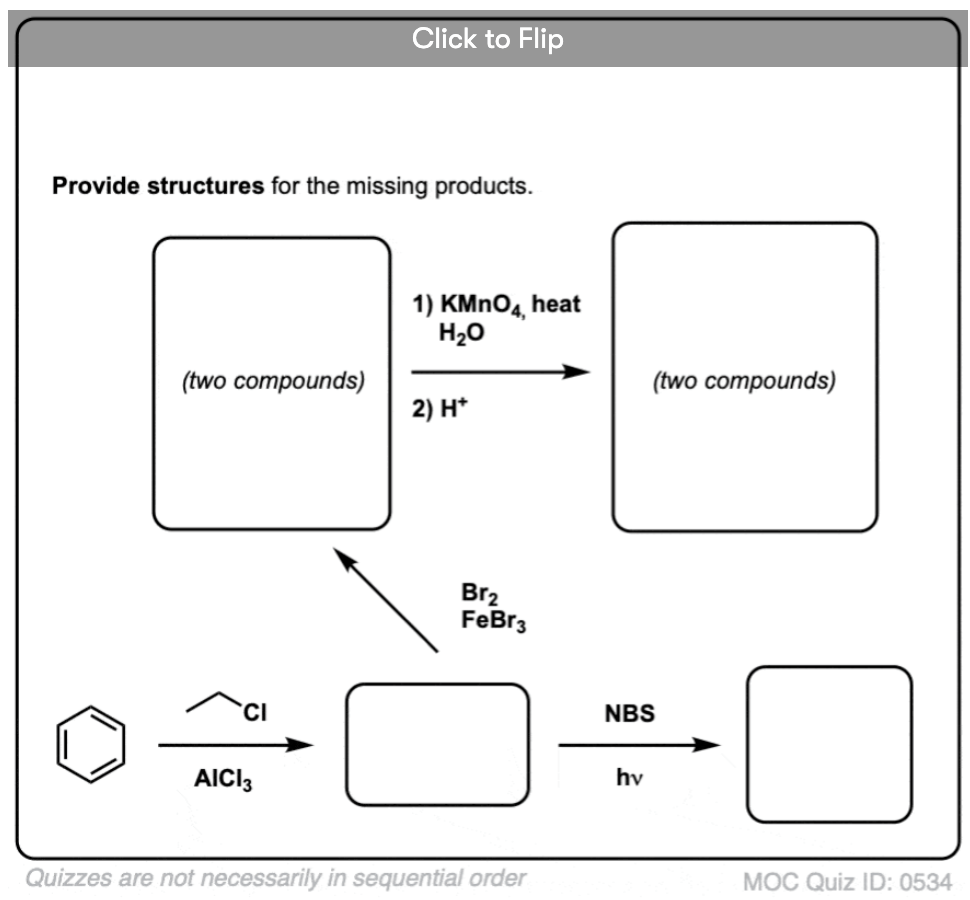
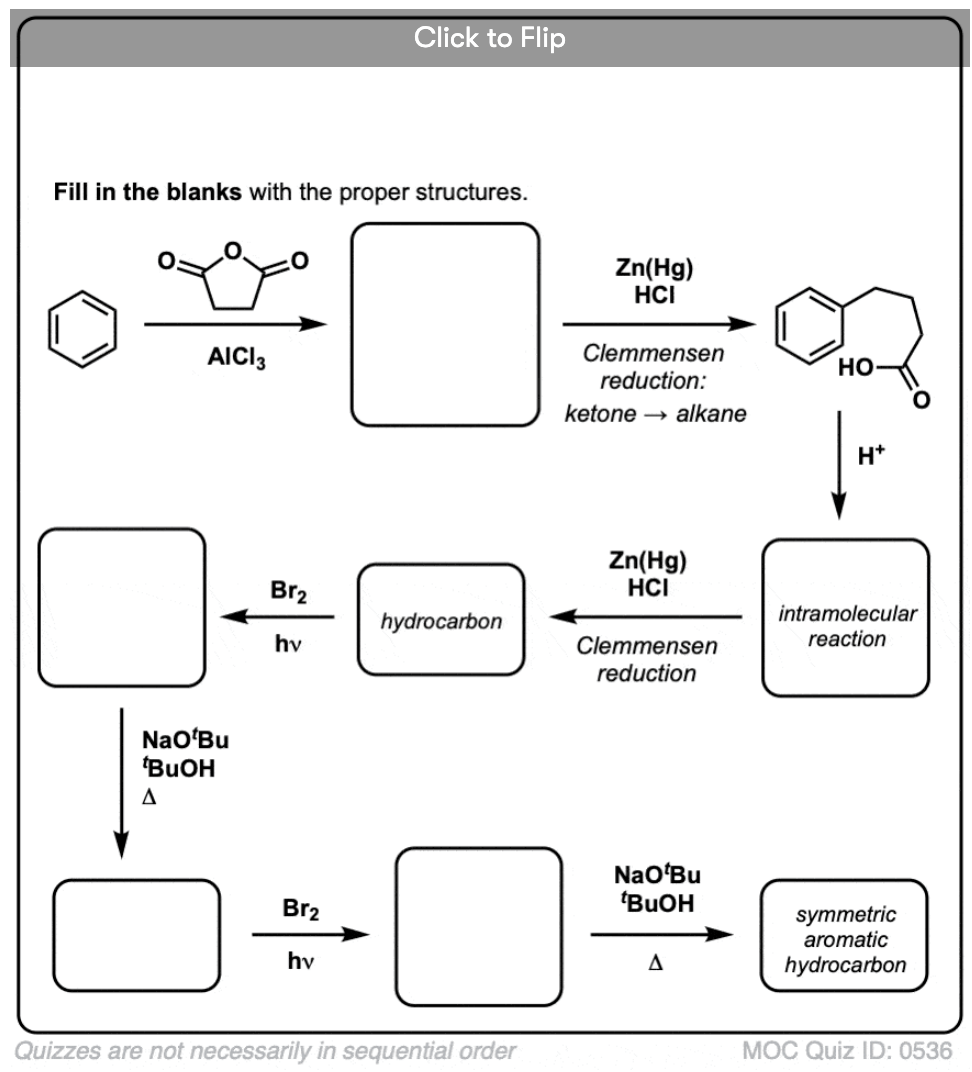
Based on the above, here’s a quick quiz for you. How might you convert tetralin (below left) to naphthalene (below right)? Answer in the footnotes [Note 3].
9. Benzylic oxidation With KMnO4 (or H2CrO4)
Since the benzylic C-H bond is relatively weak, you might rightly ask if other types of reactions (besides bromination) can be performed at this position.
They certainly can! A prime example is benzylic oxidation – where a benzylic C-H bond is broken and a benzylic C-O bond is formed.
Now: there exist fairly gentle reagents for benzylic oxidation that are the chemical equivalent of tickling the C-H bond off with a feather, resulting in a benzylic aldehyde or ketone [the alcohol is an intermediate, but usually oxidized up to the aldehyde/ketone under these conditions].
That is not the type of benzylic oxidation that is covered in a typical introductory organic chemistry course!
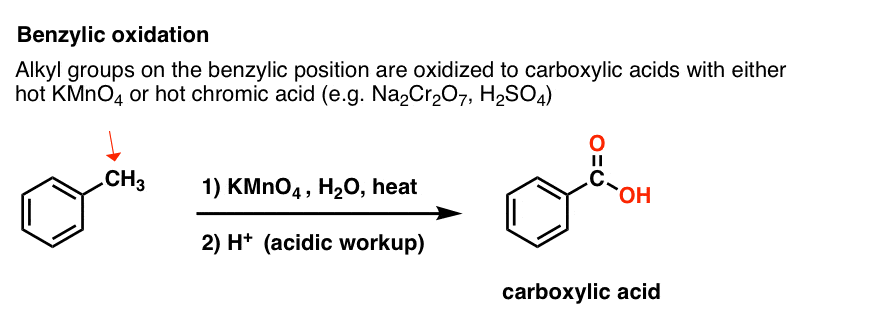
Instead, the main reagent for benzylic oxidation that is covered in most intro textbooks is hot potassium permanganate (KMnO4). Hot chromic acid (H2CrO4, which is formed by combining a dichromate salt like Na2Cr2O7 with a strong acid like H2SO4) can also be used for these purposes.
KMnO4 is not subtle. KMnO4 is not gentle. KMnO4 is not kind.
A solution of hot KMnO4 is like a bag full of angry piranhas that will tear all the flesh off of your molecule and leave only bones behind. (Come to think of it, it’s kind of like your liver! [note 4]
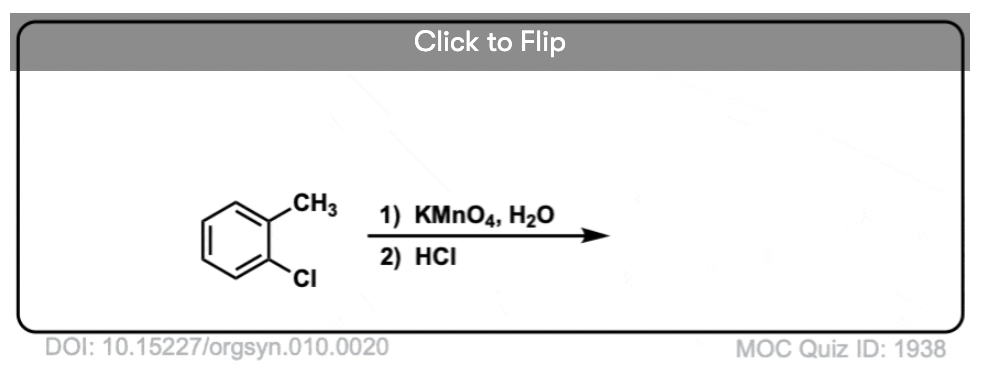
Hot KMnO4 will break every benzylic C-H bond and convert it into a C-O bond. From toluene, for example, the product is benzoic acid (a carboxylic acid).
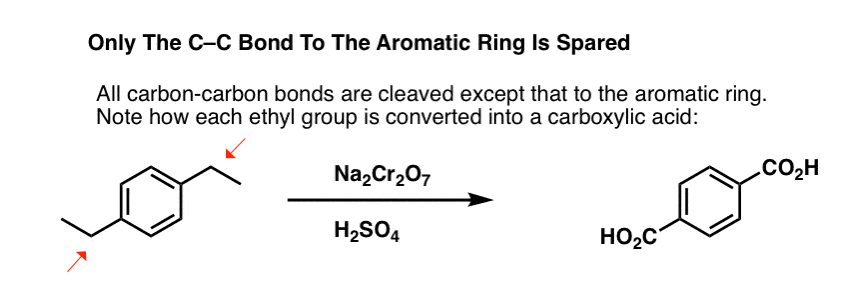
But it doesn’t just break the benzylic C-H bonds. Oh no. It goes after C–C bonds too; at least the ones that aren’t directly attached to a phenyl ring. For example, para-diethylbenzene is converted into para-benzene-dicarboxylic acid.
Note that the alkyl C-C bond has to be cleaved off in order for this oxidation to occur! (it is likely oxidized all the way up to CO2 gas).
An interesting variation is on a cyclic molecule like tetralin; the result is the dicarboxylic acid.
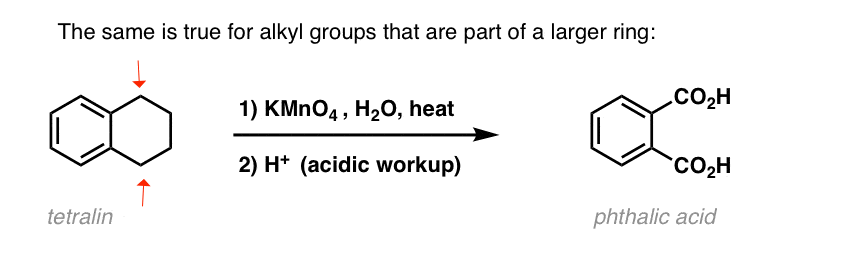
This dicarboxylic acid is called phthalic acid.
10. Benzylic Oxidation Requires A Benzylic C-H Bond
As with benzylic bromination, no reaction occurs if there is not a C-H bond on the benzylic position. For example, the reaction fails completely with t-butylbenzene:
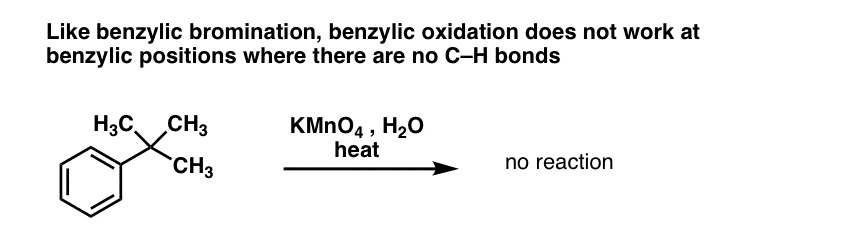
The full mechanism of benzylic oxidation is too complex, lengthy, and uncertain to show here, but the first step is almost certainly homolytic cleavage of the C-H bond by the Mn=O to form the benzylic radical.
11. Why Is This Important? [Hint: Applications In Synthesis]
Benzylic oxidation with KMnO4 is a harsh reaction, but it can have its uses. It works best with simple molecules that don’t have delicate functional groups and can withstand some very rough treatment.
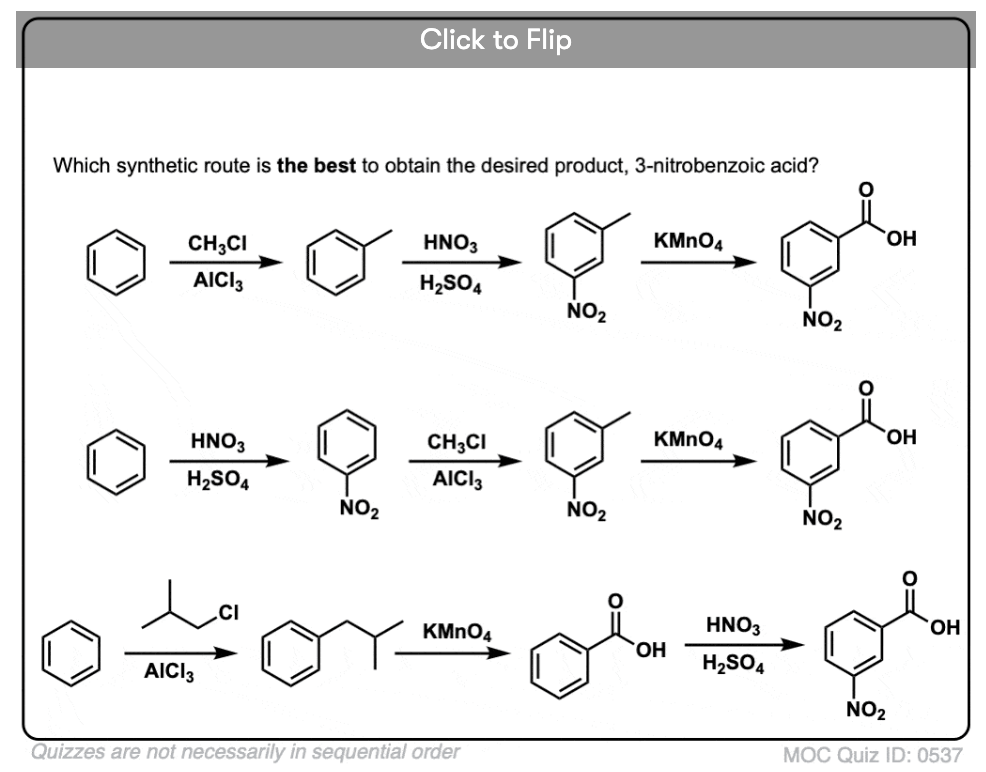
The first thing to note is that carboxylic acids can’t be placed directly on a benzene ring via the Friedel-Crafts acylation, so alkylation followed by oxidation is a useful workaround.
The second useful function in synthesis is the fact that benzylic oxidation converts an o- p- director (an alkyl group) into a meta-director.
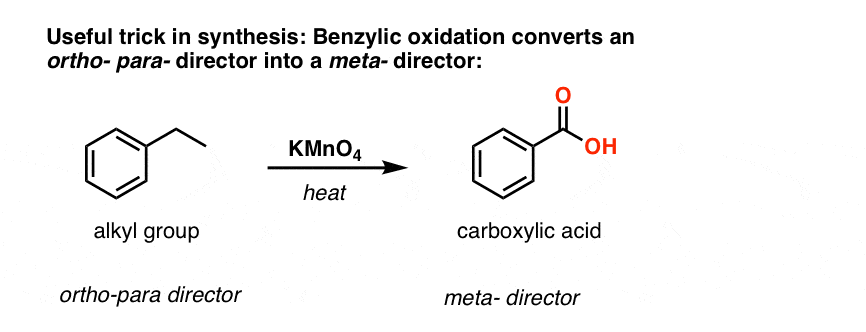
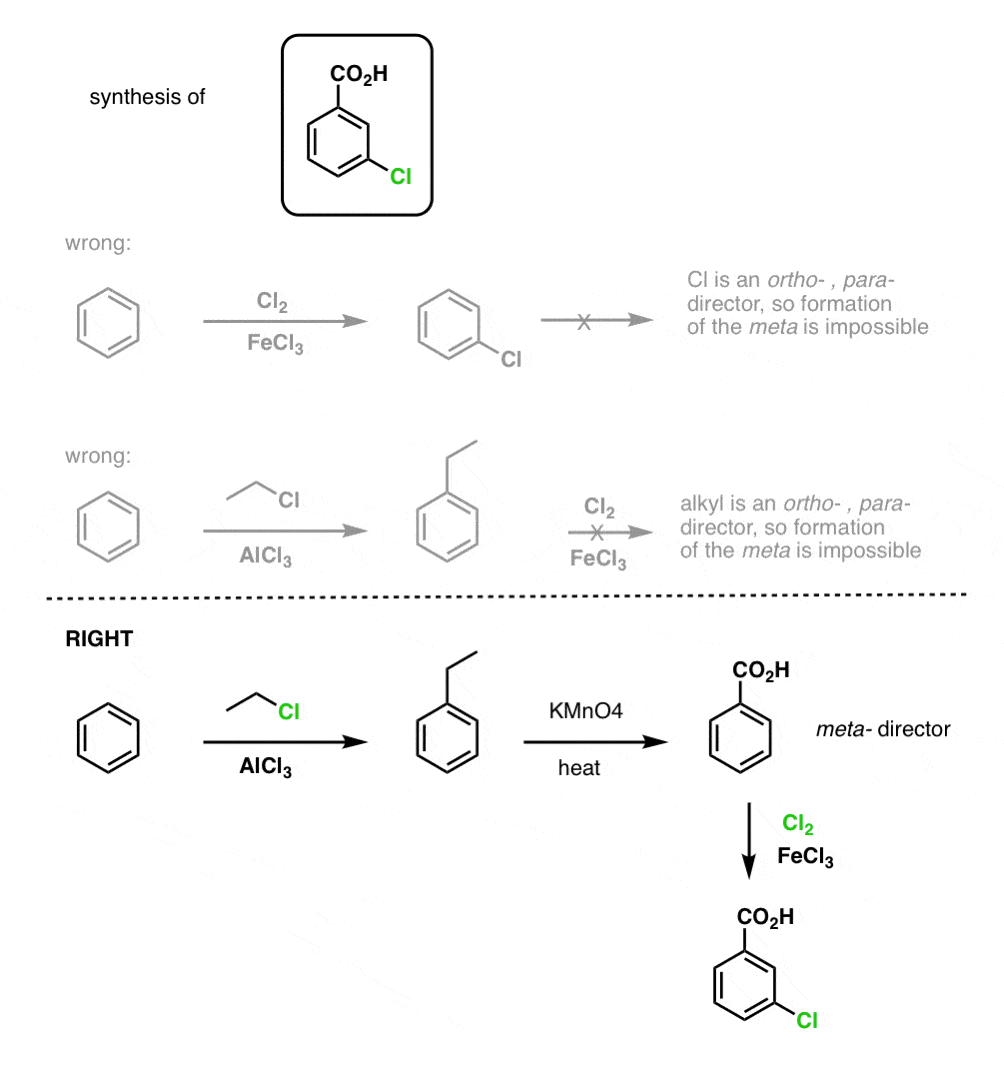
For example, confronted with the synthesis of the following molecule, how do you proceed? (hint: it involves a benzylic oxidation at some point). [answer in footnotes]
We’ll have a lot more to say about synthesis of aromatic compounds in a few more posts.
Next: Reduction of benzylic carbonyls – the Clemmensen and Wolff-Kishner reductions
Notes
Note 1. “Cabbage” can also be used as a slang term in chemistry for “all that stuff on the molecule that you can safely ignore” for whatever reason. For instance, bryostatin is a beast of a molecule that shows promise as an anticancer agent. Trouble is, it takes a ton of a rare bryozoan to get a decent amount of bryostatin, and that isn’t enough for a clinical trial on humans. So several groups have been working on making simpler, easier to synthesize “bryologs” where they get rid of the “cabbage” that isn’t essential for antitumor activity.
Note 2. Br2 will generally not react with aromatic rings without a Lewis acid catalyst. However if a strong activating group is present (such as OH, NR2, etc) some bromination of the ring can occur, especially at elevated temperatures.
Note 3. This requires two oxidations and two eliminations. A cautious way to do it is to oxidize (i.e. brominate) once, and then eliminate to give an alkene. The alkene could then be subjected to bromination again (using NBS, so as to avoid bromination of the double bond) to give another bromination (benzylic bromination product shown here, but allylic also feasible) which could be treated with base to give aromatic naphthalene.
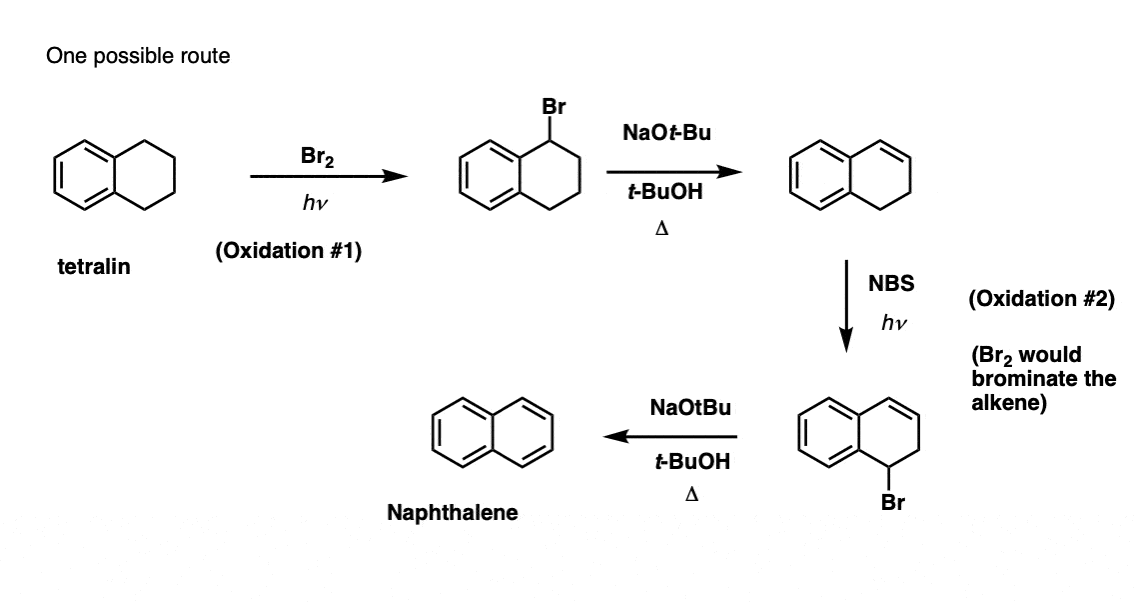
(in practice, it turns out that both brominations can be done at the same time using Br2 at high temperature. Treatment with base can then yield naphthalene).
Note 4. Fun fact: “Benzylic oxidation” is the answer to the puzzle of why methylbenzene (toluene) is not nearly as potent a carcinogen as benzene. That’s because your liver has cytochrome p450 enzymes (containing high oxidation-state iron species) that can oxidize the benzylic CH3 group to the more water-soluble carboxylic acid, allowing it to be cleared by your kidneys.
Note 5. Answer to quiz question 2.
Quiz Yourself!
Become a MOC member to see the clickable quiz with answers on the back.
Become a MOC member to see the clickable quiz with answers on the back.
Become a MOC member to see the clickable quiz with answers on the back.
Become a MOC member to see the clickable quiz with answers on the back.
Become a MOC member to see the clickable quiz with answers on the back.
Become a MOC member to see the clickable quiz with answers on the back.
Become a MOC member to see the clickable quiz with answers on the back.
Become a MOC member to see the clickable quiz with answers on the back.
(Advanced) References and Further Reading
Benzylic Bromination:
This reaction is also known as the Wohl-Ziegler reaction.
- Bromierung ungesättigter Verbindungen mit N‐Brom‐acetamid, ein Beitrag zur Lehre vom Verlauf chemischer Vorgänge
Wohl
Chem. Ber. 1919, 52 (1), 51-63
DOI: 10.1002/cber.19190520109 - Die Halogenierung ungesättigter Substanzen in der Allylstellung
Ziegler, A. Späth, E. Schaaf, W. Schumann, E. Winkelmann
Just. Lieb. Ann. Chem. 1942, 551 (1), 80-119
DOI: 10.1002/jlac.19425510103
The first two publications on this reaction. Wohl used N-bromoacetamide as the brominating agent, not NBS. Ziegler then carried out a detailed study and reported that NBS could be used as a convenient brominating agent for allylic bromination. - Dibenzo[a,e]cyclooctene: Multi-gram Synthesis of a Bidentate Ligand
Géraldine Franck, Marcel Brill, and Günter Helmchen
Org. Synth. 2012, 89, 55-65
DOI: 10.15227/orgsyn.089.0055 - Benzylic Brominations with N-Bromosuccinimide in (Trifluoromethyl)benzene
Diana Suarez, Gilles Laval, Shang-Min Tu, Dong Jiang, Claire L. Robinson, Richard Scott, Bernard T. Golding
Synthesis 2009 (11): 1807-1810
DOI: 1055/s-0029-1216793
Reference #3 features a typical benzylic bromination using NBS in CCl4, which has been largely phased out due to toxicity concerns. Reference #4 shows that PhCF3 can be used as a solvent in place of CCl4 for benzylic bromination. - Brominations with N-Bromosuccinimide and Related Compounds. The Wohl-Ziegler Reaction.
Carl Djerassi
Chemical Reviews 1948, 43 (2), 271-317
DOI: 10.1021/cr60135a004
An old review on this reaction by noted chemist Carl Djerassi, whose major contribution to global health was the development of norethindrone – the first female contraceptive. - N-Bromosuccinimide. III. Stereochemical Course of Benzylic Bromination
H. J. Dauben Jr. and Layton L. McCoy
Journal of the American Chemical Society 1959, 81 (20), 5404-5409
DOI: 10.1021/ja01529a038
A mechanistic study on the stereochemistry of benzylic bromination. By observing the formation of racemic benzylic bromides from prochiral substrates, the intermediacy of radicals in this reaction is further strengthened.The same references from allylic bromination can be repurposed here: - Mechanisms of Benzylic Bromination
Glen A. Russell, Charles. DeBoer, and Kathleen M. Desmond
Journal of the American Chemical Society 1963 85 (3), 365-366
DOI: 1021/ja00886a040
Benzylic bromination follows the same mechanism as allylic bromination, as this paper explains.NBS (N-Bromosuccinimide) is a convenient reagent for free-radical bromination, and the following papers are mechanistic studies involving NBS: - The Mechanism of Benzylic Bromination with N-Bromosuccinimide
R. E. Pearson and J. C. Martin
Journal of the American Chemical Society 1963 85 (3), 354-355
DOI: 10.1021/ja00886a029
These papers by Prof J. C. Martin (UIUC) were early in his career, before he did the work that he is most well-known for (studies on ‘hypervalent’ molecules, including the development of the ‘Dess-Martin Periodinane’). - The Identity of the Chain-Carrying Species in Brominations with N-Bromosuccinimide: Selectivity of Substituted N-Bromosuccinimides toward Substituted Toluenes
R. E. Pearson and J. C. Martin
Journal of the American Chemical Society 1963, 85 (20), 3142-3146
DOI: 10.1021/ja00903a021 - N-bromosuccinimide. Mechanisms of allylic bromination and related reactions
J. H. Incremona and James Cullen Martin
Journal of the American Chemical Society 1970, 92 (3), 627-634
DOI: 10.1021/ja00706a034 - Succinimidyl radical as a chain carrier. Mechanism of allylic bromination
J. C. Day, M. J. Lindstrom, and P. S. Skell
Journal of the American Chemical Society 1974, 96 (17), 5616-5617
DOI: 10.1021/ja00824a074 - Radical Bromination of Cyclohexene in CCl4 by Bromine: Addition versus Substitution
D. W. McMillen and John B. Grutzner
The Journal of Organic Chemistry 1994, 59 (16), 4516-4528
DOI: 10.1021/jo00095a029
This paper describes careful kinetic studies that demonstrate that a low concentration of Br2 (such as that provided by impure NBS) will favor radical substitution over a polar addition reaction. - o-XYLYLENE DIBROMIDE
Emily F. M. Stephenson
Org. Synth. Vol. 34, p.100 (1954)
DOI: 10.15227/orgsyn.034.0100
A reliable procedure for benzylic bromination with Br2 in Organic Syntheses. - Bromination of Tetralin. Short and Efficient Synthesis of 1,4-Dibromonaphthalene
Osman Çakmak, Ismail Kahveci, Íbrahim Demirtaş, Tuncer Hökelek and Keith Smith
Czech. Chem. Commun. 2000, 65, 1791-1804
DOI: 10.1135/cccc20001791
A convenient synthesis of 1,4-dibromonaphthalene from tetralin, via benzylic bromination.Benzylic oxidation:
A lot of strategies exist for benzylic oxidation, including the use of concentrated solutions of KMnO4 or chromic acid. - Kinetic studies of the oxidation of aromatic compounds by potassium permanganate. Part IV. n- and iso-Propylbenzene
C. F. Cullis and J. W. Ladbury
J. Chem. Soc. 1955, 4186-4190
DOI: 10.1039/JR9550004186 - PIPERIDINE DERIVATIVES X. THE PHENYLPIPERIDYLCARBINOLS
Kenneth E. Crook and S. M. McElvain
Journal of the American Chemical Society 1930, 52 (10), 4006-4011
DOI: 1021/ja01373a035
If there is another substituent on the benzylic position, then oxidation with give a ketone instead of a carboxylic acid. - Heterogeneous Permanganate Oxidations. 7. The Oxidation of Aliphatic Side Chains
Nazih A. Noureldin, Dongyuan Zhao, and Donald G. Lee
The Journal of Organic Chemistry 1997, 62 (25), 8767-8772
DOI: 1021/jo971168e
Reagents can be adsorbed onto solid supports to make reactions easier to conduct and reduce waste. In this case, by making the KMnO4 reagent heterogenous, the reactivity changes slightly, and this can be exploited for some chemoselective oxidations.A lot of other reagents can be used for benzylic oxidation, here is a selection: - Selective Oxidation at Carbon Adjacent to Aromatic Systems with IBX
K. C. Nicolaou, Phil S. Baran, and Yong-Li Zhong
Journal of the American Chemical Society 2001, 123 (13), 3183-3185
DOI: 10.1021/ja004218x
An early paper from (now rockstar of total synthesis) Prof. Phil Baran (The Scripps Research Institute, La Jolla, CA) while he was still a PhD student in Prof. Nicolaou’s group. This uses IBX (a hypervalent I(V) reagent) to do benzylic oxidations, which occur via a radical mechanism. This is a useful reaction with broad substrate scope, as exemplified by 25 examples in the paper. - Direct and Selective Benzylic Oxidation of Alkylarenes via C–H Abstraction Using Alkali Metal Bromides
Katsuhiko Moriyama, Misato Takemura, and Hideo Togo
Organic Letters 2012, 14 (9), 2414-2417
DOI: 10.1021/ol300853z
This uses Oxone as the terminal oxidant for benzylic oxidation. - Synthesis of Benzylic Alcohols by C−H Oxidation
Lalita Tanwar, Jonas Börgel, and Tobias Ritter
J. Am. Chem. Soc. 2019, 141, 17983-17988
DOI: 10.1021/jacs.9b09496
This uses mesyl peroxide as an oxidant for the selective oxidation of benzylic positions to alcohols. The benzyl mesylate is formed, which can be hydrolyzed to the alcohol. - Electrochemical benzylic oxidation of C–H bonds
Jason A. Marko, Anthony Durgham, Stacey Lowery Bretz, and Wei Liu
Chem. Commun., 2019, 55, 937-940
DOI: 10.1039/C8CC08768G
Benzylic oxidation can also be effected through electrochemistry. - Allylic and benzylic oxidation reactions with sodium chlorite
Samuel M. Silvestre, Jorge A. R. Salvador
Tetrahedron 2007, 63 (11), 2439-2445
DOI: 10.1016/j.tet.2007.01.012
NaClO2 is inexpensive and can also be used as an oxidant.
Hi James,
I have one coment to the last question in your quiz. You mentioned that the best synthetic route to get m-nitrobenzoic acid is the second one. It is possible to provide FC alkylation even on the relative electron-poor nitrobenzen (compared to e.g. benzen)? Because, some textbooks (such as e.g., McMurry) mentions that (in general) this type of reaction is not carried out. Thank you for your coment.
The last route is wrong because you can´t get n-propyl/isopropyl benzen using methyl chloride.
What happens with the carbons? I was thinking, what if we took a dimethyl substituted version of tetralin where one of the benzylic positions had both its H’s replaced by methyl groups, then oxidized it? That position would be unaffected in the reaction, but the other position would turn into a carboxylic acid, and I imagine the chain would unravel. But what would be at the end of that chain?
Hi Robert – The molecule would have two carboxylic acids, one at the benzylic position and one at the end of the chain (on the carbon 2 carbons over from the dimethylated benzylic carbon).
Definitely a worthy topic to discuss. Thank you, James. A couple of notes though. For the fairness sake, I have to note, that when the benzylic hydrogen is not available, the radical brominatiln with NBS is still quite possible, won’t give you the substitution at benzylic position, but still the reaction does occur, so it might be misleading saying that it fails completely and there’s no reaction. When it’s done with t-Bu-Ph, it also gives a radical rearrangement that in fact does yield the benzylic radical.
Also, I believe you’ve mentioned in one of your previous posts that FC alkylation often yields multiple alkylation products and is generally not a good reaction for the actual synthetic purposes, yet you use it here as the first step in your synthesis. Tut-tut ?
Thanks Victor.
1) I was not previously aware of the neophyl radical rearrangement. Thank you.
2) Guilty as charged re: the FC alkylation. Good point.
I wonder if it is possible to obtain a good yield of mono alkylation product by using a large excess of benzene? A professor claimed that in at least one class. In that case the reaction might work fine for simple synthesis like making toluene.
But vast majority of professors would accept monalkylation to make toluene. Otherwise you would need to write “obtain by distillation from hydrocarbons obtained through fracking”. Or “buy from Aldrich.”
Or something else awkward like formylation/reduction or carboxylation/reduction. Or organometallic transformation that few students have learned.
I’ve read that the radical bromination of primary carbon isn’t as effective as the corresponding chlorination. With the fairly low concentration of Br2 created by NBS, which result in an even lower concentration of Br radical, is it possible for NBS to brominate the primary carbons? For example in such compounds as t-butyl benzene, ethane, & neo-pentane?
As usual, excellent post. Some minor corrections are needed, though:
– “here’s are the products” (under “Benzylic Bromination”);
– in the scheme under “When Does It NOT Work?” a 3 is missing in the structure (CH instead of CH3);
– “So why might allylic bromination be useful?” – it should read “benzylic bromination”;
– “Benzylic oxidation With KMnO4 (or H2CrO4)” – it should be “Benzylic Oxidation” (all right, that’s a really small one);
– under this title: “oxidized up to the alcohol/ketone” – it should read “aldehide/ketone”.
Thank you very much.