Enols and Enolates
Claisen Condensation and Dieckmann Condensation
Last updated: March 24th, 2025 |
The Claisen Condensation (And Its Intramolecular Version, The “Dieckmann” Condensation)
- The Claisen condensation is one of the fundamental reactions of esters.
- In the Claisen condensation, an ester (2 equivalents) is treated with a base (1 equivalent); the product is a called “beta-keto” ester, since a ketone is located two carbons away (beta) from the ester carbonyl.
- It forms a new C-C bond and breaks a C-O and C-H bond.
- The Dieckmann condensation is the name given to an intramolecular Claisen condensation that results in a 5- or 6-membered ring.
Here’s a specific example:
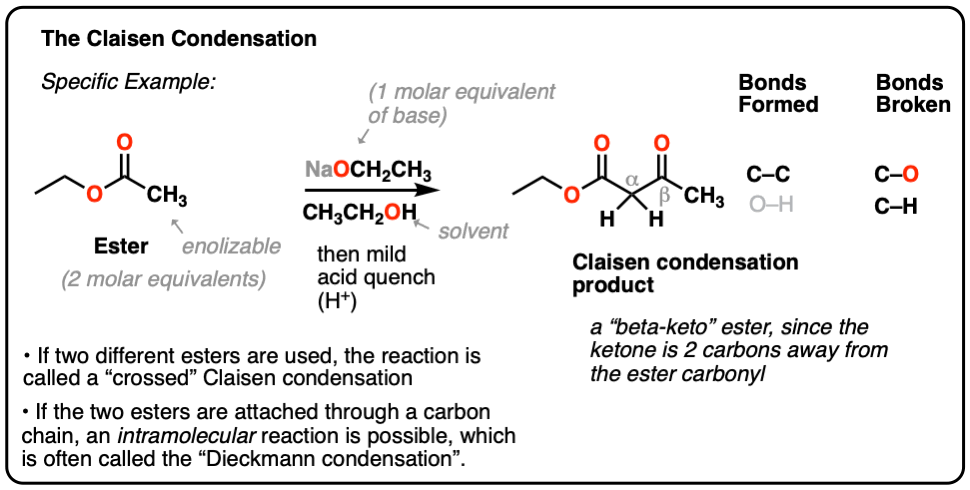
When two different esters are involved, the reaction is often called a “crossed” Claisen.
Like many reactions, the Claisen has an intra-molecular variant, which goes by the name of the Dieckmann condensation, even though it is still the same reaction and goes through the same mechanism. Different nameplate on the car, same engine under the hood.
The mechanism is important to learn since it incorporates so many of the key reactive properties of esters – deprotonation to give enolates, how those enolates can act as nucleophiles, and how the ester carbonyl can undergo addition-elimination reaction (i.e. nucleophilic acyl substitution)
In this post we’ll show each step of the Claisen condensation mechanism in detail, along with examples of crossed Claisen condensations and Dieckmann condensations. There are quiz questions at the end that will test you on some of the key concepts.
Table of Contents
- The Claisen Condensation
- Formation Of Ester Enolates Through Deprotonation With Strong Base
- Nucleophilic Addition and Elimination To Carbonyls (a.k.a. Nucleophilic Acyl Substitution)
- Deprotonation Of The Beta-Keto Ester Product
- Crossed Claisen Condensations
- Intramolecular Claisen Condensations – The Dieckmann Reaction
- Conclusion: Claisen and Dieckmann
- Notes
- Quiz Yourself!
- (Advanced) References and Further Reading
1. The Claisen Condensation
When an ester (2 molar equivalents) is treated with a strong base (one molar equivalent), a new product is obtained, with the key outcome being that a new C-C bond is formed and a C-H bond and C-O bond are broken.
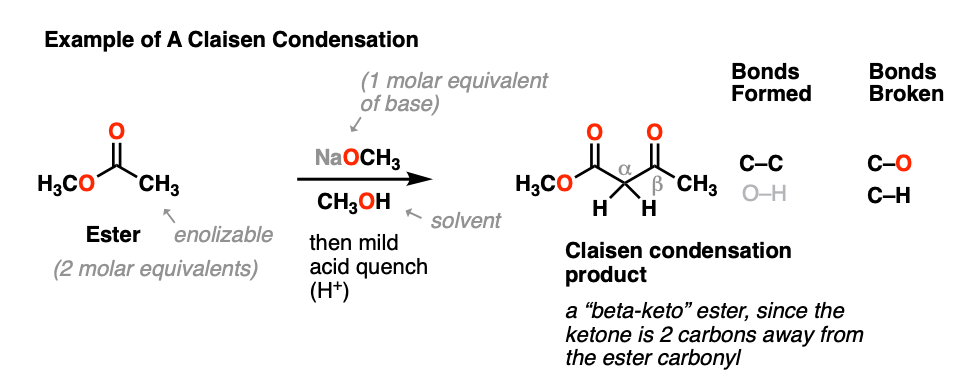
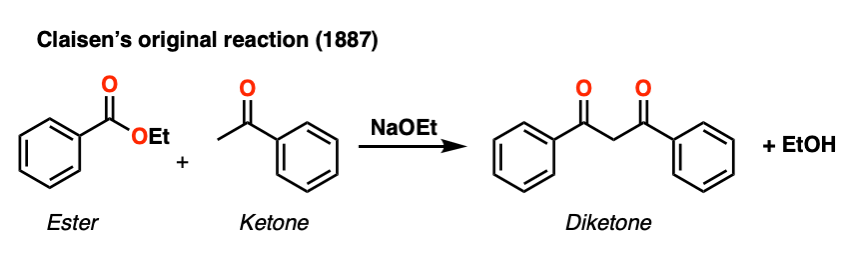
This general process has come to be known as the Claisen condensation, after Ludwig Claisen, a 19th century German chemist (of Claisen rearrangement and Claisen adapter fame) who reported the reaction in 1887. [Note 1]
This reaction is a way of joining two esters together via a new C-C bond to give a new product known as a “beta-keto” ester, so named because a ketone resides two carbons away (“beta”) to the ester carbonyl.
Of course, focusing on the key outcome (forming C-C, breaking C-H and C-O) is like beginning a movie with its final scene. The question is, “how did we get there”?
As we’ll see, the mechanism of the Claisen is a grand tour through some of the key features of esters, such as their reaction with bases to give enolates, how those enolates act as nucleophiles, how the ester carbonyl can undergo addition-elimination reactions with nucleophiles, and finally how the resulting products (beta-keto esters) end up considerably more acidic than the starting esters, with a thermodynamic sink thrown in for good measure.
2. Formation Of Ester Enolates Through Deprotonation With Strong Base
The carbon adjacent to a carbonyl is called the “alpha-carbon”. When the alpha carbon has C-H bonds, it is said to be enolizable, since deprotonation will result in a species called an enolate. The alpha-carbons of ketones, esters, and other related molecules are orders of magnitude more acidic than the C-H bonds of alkanes since the resulting negative charge on carbon can be delocalized to oxygen via resonance.
The pKa of the alpha-carbon of esters are about 25, which makes them about 106 to 107 less acidic than ketones (pKa about 18). When treated with a strong base like NaOCH3, deprotonation of the alpha carbon can occur, resulting in its enolate.
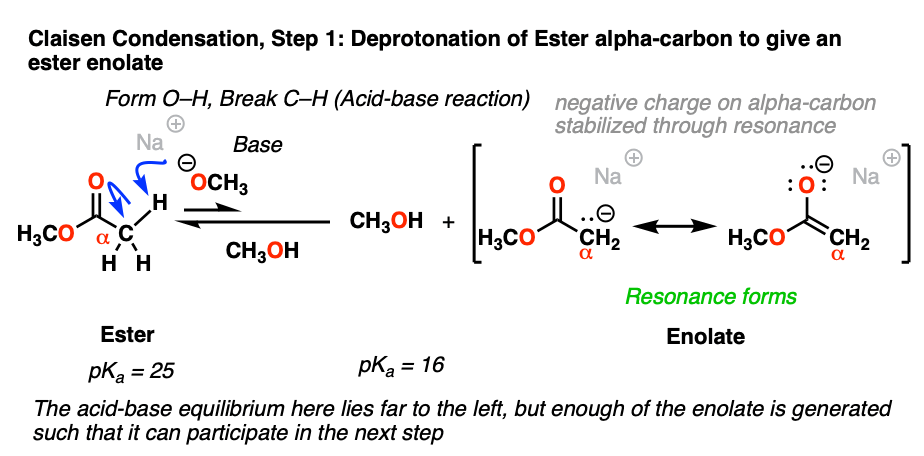
Even though the equilibrium favors the starting material vs. the enolate product by a factor of 8 pKa units (that is, about 108 :1) , the resulting enolate is reactive enough in the subsequent addition step that eventually all of the starting ester is converted to the final product.
The alkoxide base (RO-) is usually chosen to match the ester -OR group. Why do you think that might be? [see quiz below]
3. Nucleophilic Addition and Elimination To Carbonyls (a.k.a. Nucleophilic Acyl Substitution)
As we’ve seen many times before, enolates are good nucleophiles, and as nucleophiles they tend to form bonds at the carbon. [Note 2]
The enolate carbon will attack the best electrophile present, which happens to be the carbonyl carbon of another equivalent of ester.
This represents by far the most important mechanism of the carbonyl group: nucleophilic addition (or, alternatively, “1,2-addition”). As the nucleophile forms a new bond with the carbonyl carbon, the carbon-oxygen pi bond breaks, producing a tetrahedral intermediate.
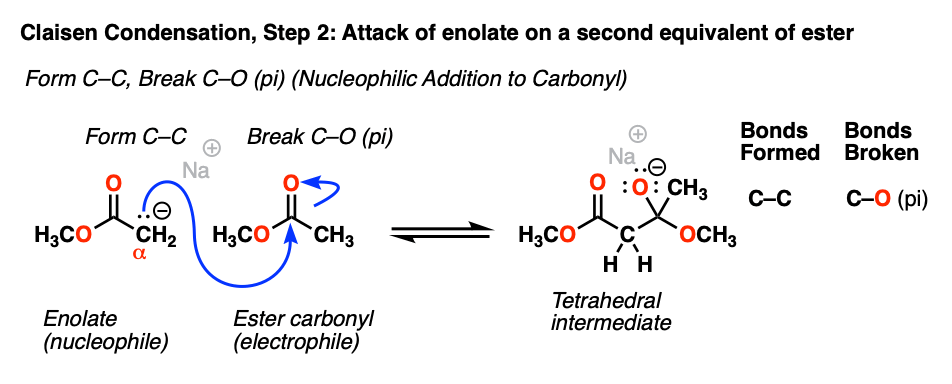
[Note that this reaction is reversible. There is an equilibrium between the tetrahedral intermediate and the starting materials (i.e. the ester enolate + ester).]
Now we come to the second-most important reaction of the carbonyl functional group: elimination (or, alternatively, 1,2-elimination). The negative charge in the oxygen of the tetrahedral intermediate can re-form the C-O pi bond, and in so doing break the C–O sigma bond. The result is a new ketone and an alkoxide (RO– ) leaving group:
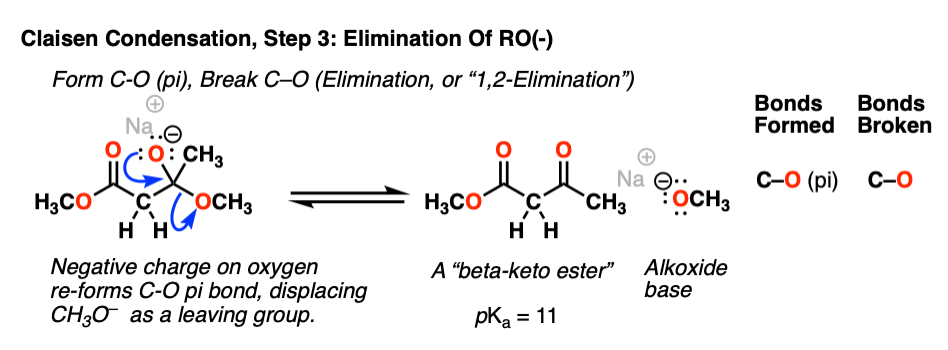
Normally one wouldn’t think of an alkoxide as a good leaving group (the pKa of ethanol is 16, making CH3CH2O– a strong base) but elimination of RO(-) is still a much more favorable pathway than reversal of the addition reaction to give the ester enolate (pKa of the ester = 25). [Note 3].
Together, steps 2 and step 3 are called, “nucleophilic acyl substitution” since the OR of the ester has been replaced with CH2CO2R.
This 1-2 punch of addition-elimination is a characteristic reaction of acyl groups (RC(O)– ) and we will see it again and again and again.
4. Deprotonation Of The Beta-Keto Ester Product
Having shown how nucleophilic acyl substitution occurs in the Claisen, our reaction mechanism is nearly complete. However, there is still one pesky loose end to tie up.
We saw that nucleophilic acyl substitution on the ester results in a new strong base, RO(-).
It so happens that the product of the Claisen, the beta-keto ester, is a considerably stronger acid (pKa 11) than the conjugate acid of RO(-), ROH (pKa 16).
As we’ve seen countless times in acid-base reactions, a stronger acid plus a stronger base will give a weaker acid and a weaker base. [See: Acid-Base Reactions In Organic Chemistry]
That means that the alkoxide base will easily deprotonate the resulting beta-keto ester, giving a new enolate – that of the beta-keto ester.
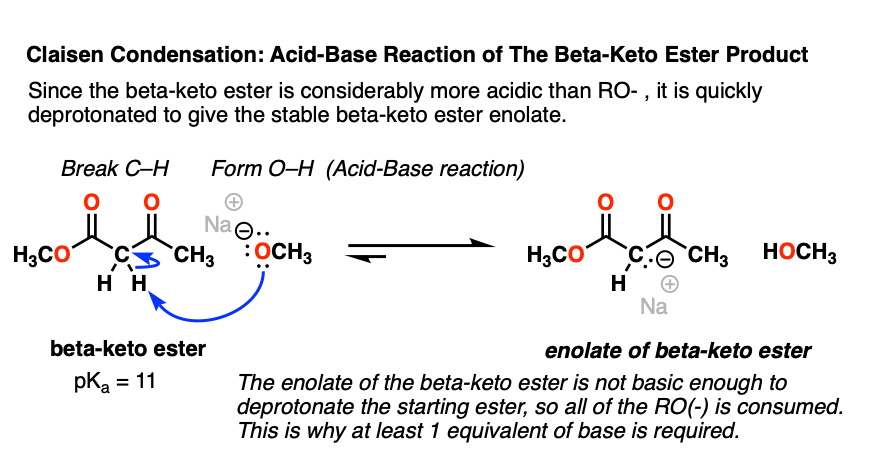
Why is the beta-keto ester (pKa 11) so acidic? Note that the negative charge on carbon can now be delocalized through carbon to two different oxygens, which are more electronegative and better able to stabilize negative charge:
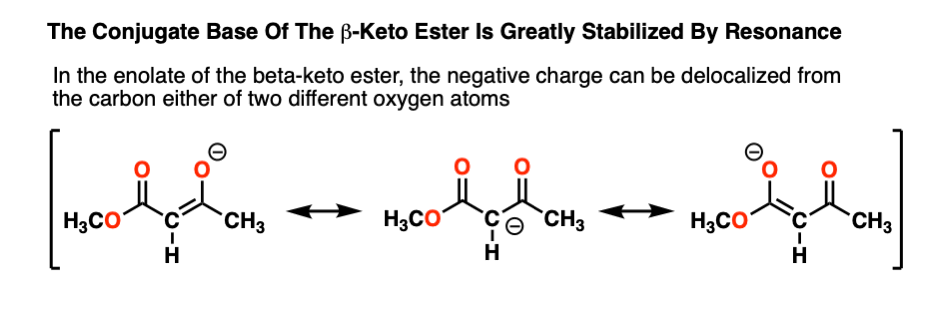
The more “spread out” a negative charge is, the more stable it is, generally speaking. “Instability of negative charge” usually correlates with high basicity, and vice-versa.
The enolate is so stable, in fact, that it represents what chemists often call a “thermodynamic sink”. Once formed, the equilibrium going back to starting materials is so unfavorable that the enolate just sits around in the reaction flask, not undergoing any significant reaction. [Note 4]
In this respect it resembles water that has flowed into a sink or bucket and must flow “uphill” to get out.Just as all rivers run to the sea, in the Claisen condensation, all our ester starting material eventually converts to the enolate of the beta-keto ester. [Note 5]
It’s worth noting that the enolate of the beta-keto ester is such a weak base that it is unable to deprotonate any of the starting ester (pKa =25). This is why that in order for the reaction to proceed, we must use at least one full equivalent of base. [This is in contrast to the Aldol reaction, by the way, where only catalytic base is required since the reaction produces RO(-) ]
Having nothing to do once formed, the enolate of the beta keto ester just sits around in the reaction flask until mild acid is added to quench the reaction.
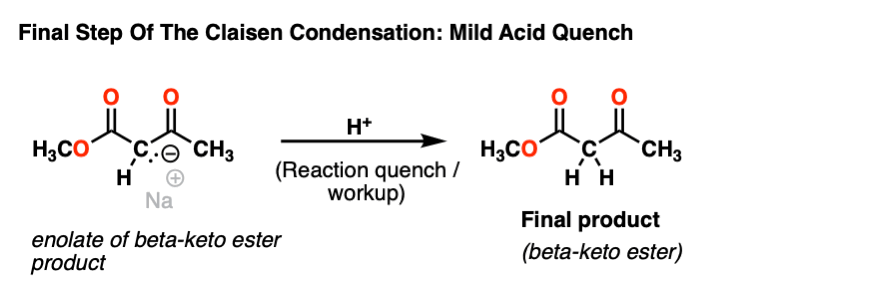
This gives us the beta-keto ester product.
5. “Crossed” Claisen Condensations
Combining two identical esters is nice, but what would be even better is to join together two different esters.
This can be done, and it’s called the “crossed” Claisen condensation. Here’s an example:
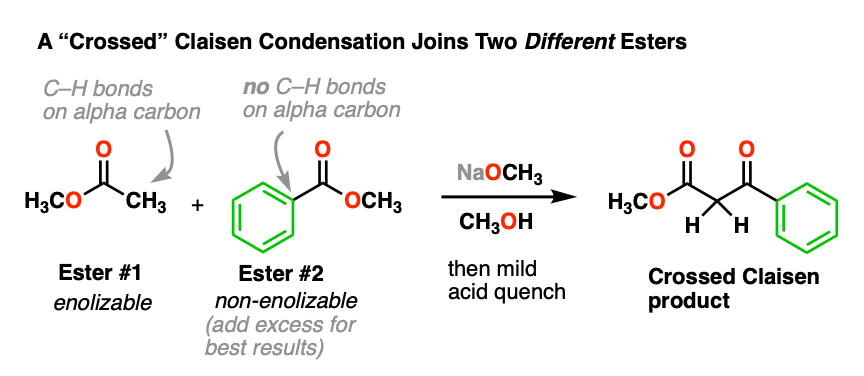
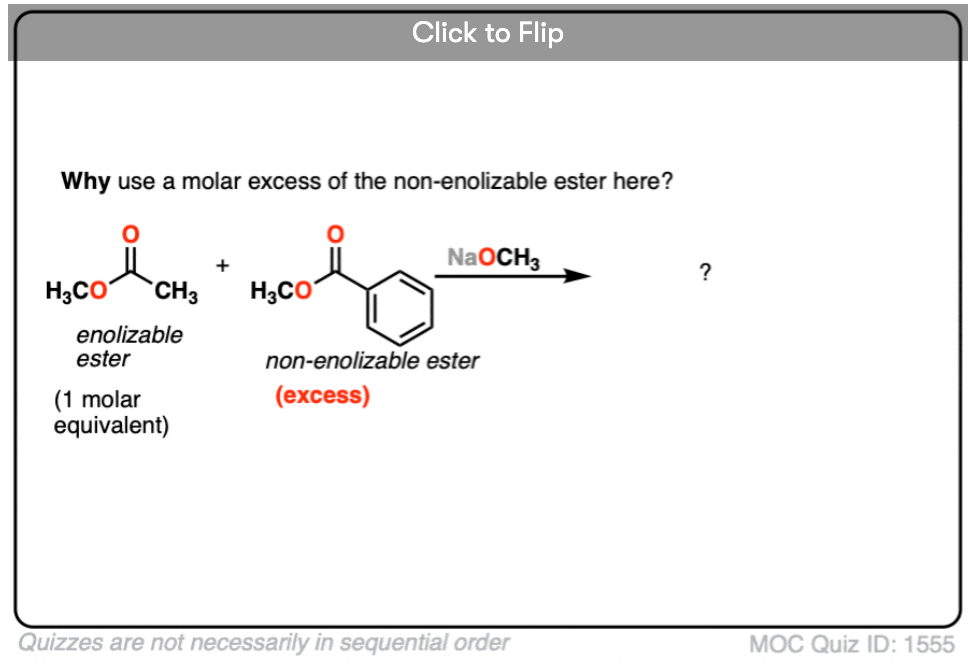
In this reaction, we have two different esters, but note that only one is “enolizable” – that is, has an alpha carbon with a C-H bond – and is therefore capable of forming an enolate. This simplifies things considerably, since only one enolate nucleophile can be formed. We can improve the chances of a crossed Claisen even more by adding an excess of the non-enolizable ester.
- Why do you think it helps to add an excess of the “non-enolizable” ester? [see quiz].
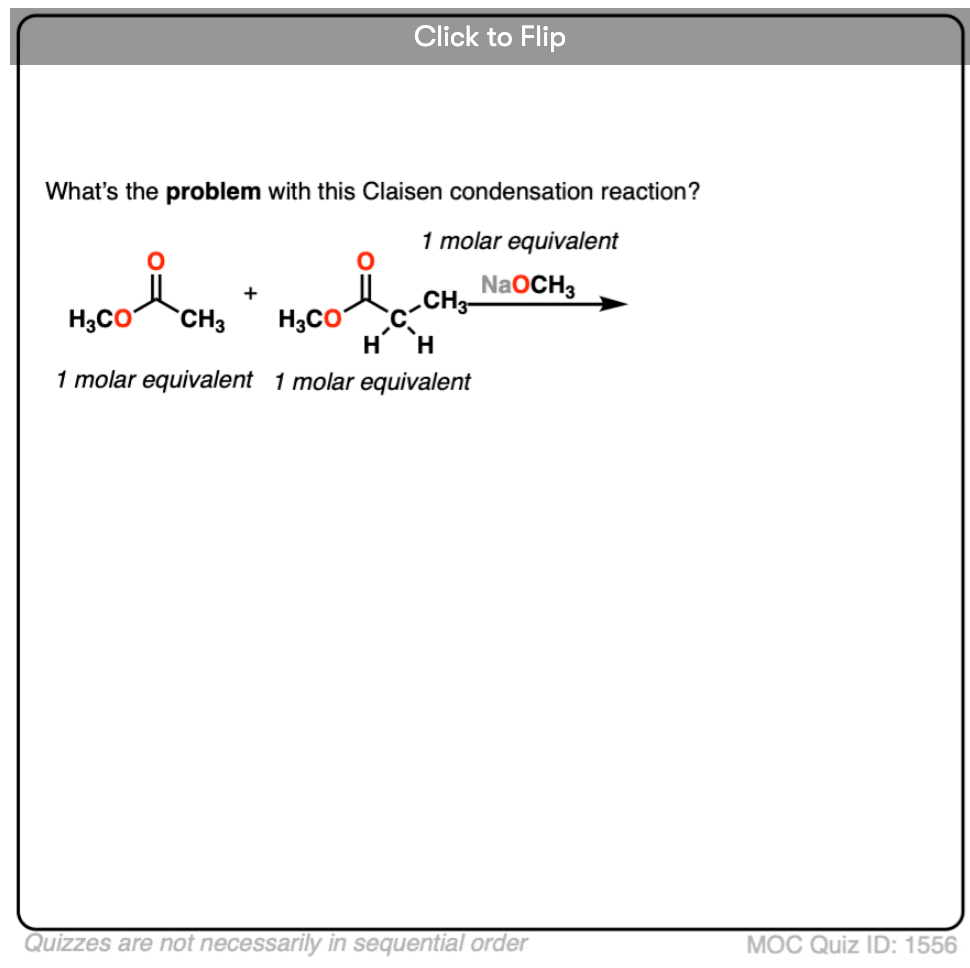
- Why might it be a bad idea to use two enolizable esters? [see quiz]
6. Intramolecular Claisen Condensations – The Dieckmann Reaction
As we’ve seen many times before, if a nucleophile and an electrophile are attached to each other through an intervening carbon chain, the result will be a new ring.
The Claisen is no different. Although for historical reasons, this version of the Claisen often goes by a different name – the Dieckmann condensation, after a certain W. Dieckmann who made a name for himself studying this reaction around the turn of the 20th century. The name isn’t important; it’s the same reaction, only intramolecular. As Shakespeare once said, a reaction by any other name would still form and break the same bonds. [Note 5]
It’s the same process we went through in the first section. First, deprotonation of an alpha-carbon to give an enolate. Second, attack of the enolate on an ester. Since the nucleophile and the electrophile are attached through a carbon chain, this results in a new ring.
Here’s an example of the Dieckmann:
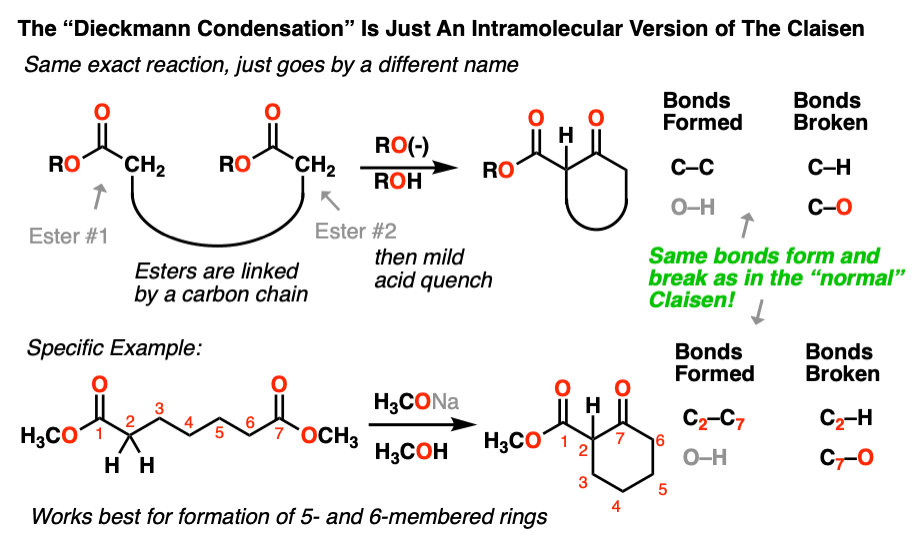
As with the other intramolecular reactions we’ve encountered, this reaction works best when we are forming five and six-membered rings.
You probably know this by now, but anytime you read an article about the “intramolecular” version of a reaction on this website, it usually means, “watch out for this on final exams”.
To see the full mechanism, hover here and an image will pop up or click this link.
7. Conclusion: Claisen and Dieckmann
Here’s what we’ve learned about the Claisen condensation:
- Ester enolates can be formed with alkoxides, and they are good nucleophiles
- Esters can undergo addition-elimination reactions with nucleophiles, otherwise known as nucleophilic acyl substitution. In the Claisen condensation the nucleophile is the ROC(O)CH2 group which displaces (RO-).
- Alkoxides can be adequate leaving groups in this case, since they’re significantly weaker bases than ester enolates.
- The product of a Claisen condensation is a beta-keto ester, which is a significantly stronger acid (pKa = 11) than either alcohols (pKa = 16-17) or esters (pKa = 25).
- The reaction requires at least one equivalent of base since the enolate of the beta-keto ester product is not a strong enough base to deprotonate the starting ester.
- A “crossed” Claisen condensation involves the enolate of one ester reacting as a nucleophile with a different (ideally non-enolizable) ester to give a beta-keto ester.
- When the enolate and ester are on the same molecule, a Claisen condensation will form a ring. This intramolecular Claisen is known as the Dieckmann condensation and works best for forming 5- and 6-membered rings.
Notes
Note 1. Although the term “Claisen condensation” in undergraduate textbooks typically refers to the reaction of an ester enolate with another ester, it was Geuther who made ethyl acetoacetate from ethyl acetate back in 1863. Claisen’s 1887 contribution was to generalize the process to other enolates; for instance Claisen’s 1887 paper has a ketone enolate reacting with an ester. [See ref below] Claisen also made significant contributions to understanding the mechanism.
Note 2. Grossman’s “second-best” rule is handy to know: “The second-best resonance structure often provides the key to understanding the chemical behavior of that compound” (from, “The Art of Writing Reasonable Organic Reaction Mechanisms“, 2nd. ed. page 9). Thus even though the “best” enolate resonance form has the negative charge on oxygen, the “second-best” (negative charge on carbon) determines the reactivity.
Note 3. The weaker the base, the better the leaving group. [See: What Makes a Good Leaving Group]. So even though we’d normally consider RO- to be a pretty bad leaving group (the conjugate base of ethanol, pKa 16) it still beats the pants off of an ester enolate (conjugate base of an ester, pKa 25) in terms of leaving group ability.
Note 4. . You might also wonder: if enolates are good nucleophiles, why doesn’t this one react with an ester carbonyl, like the ester enolate does? Some addition to an ester carbonyl probably does occur, but after nucleophilic attack, the tetrahedral intermediate (stronger base) would quickly revert back to the starting enolate (weaker base) + ester. Since all the steps are in equilibrium, and the beta-keto ester enolate is the weakest base of all the species in equilibrium, the beta-keto ester enolate is the “thermodynamic sink” in this reaction.
Note 5. In fairness to Dieckmann, beyond his contribution to the development of the intramolecular version of the Claisen condensation, he also made the important discovery of general reversibility of the Claisen reaction. [See Ingold, p. 1173, for a helpful account]
The reversibility of the reaction becomes more important as the alpha-carbon becomes increasingly more substituted. It’s interesting to note that the Claisen doesn’t work in cases where the beta-keto ester can’t enolize. Like this one:
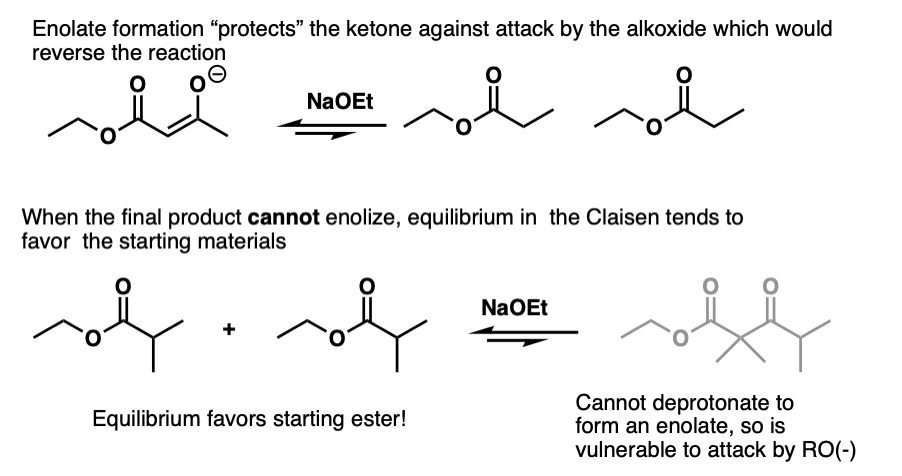
The problem isn’t that the Claisen can’t happen, it’s that the final product can undergo the reverse reaction and bust open the product.
Formation of the enolate therefore helps to “protect” the ketone against attack by nucleophiles not only thermodynamic from an acid-base perspective but also helps to protect against attack by nucleophiles.
Quiz Yourself!
1. In the Claisen condensation reactions we’ve seen here, the base (RO-) is identical to the RO group on the ester. What would happen if they were different?
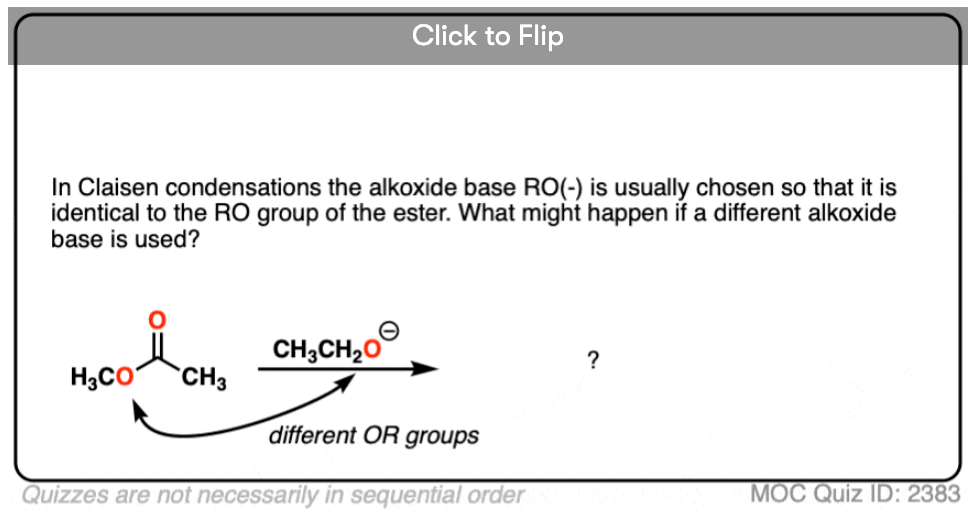
Become a MOC member to see the clickable quiz with answers on the back.
2. In the crossed Claisen reaction we showed, only one ester was enolizable, and the non-enolizable ester was added in excess. Why use the non-enolizable ester in excess?
Become a MOC member to see the clickable quiz with answers on the back.
3. What could happen if we used two enolizable esters in equal amounts?
Become a MOC member to see the clickable quiz with answers on the back.
4. Can you think of a work-around to successfully do a crossed Claisen such that only one product is obtained?
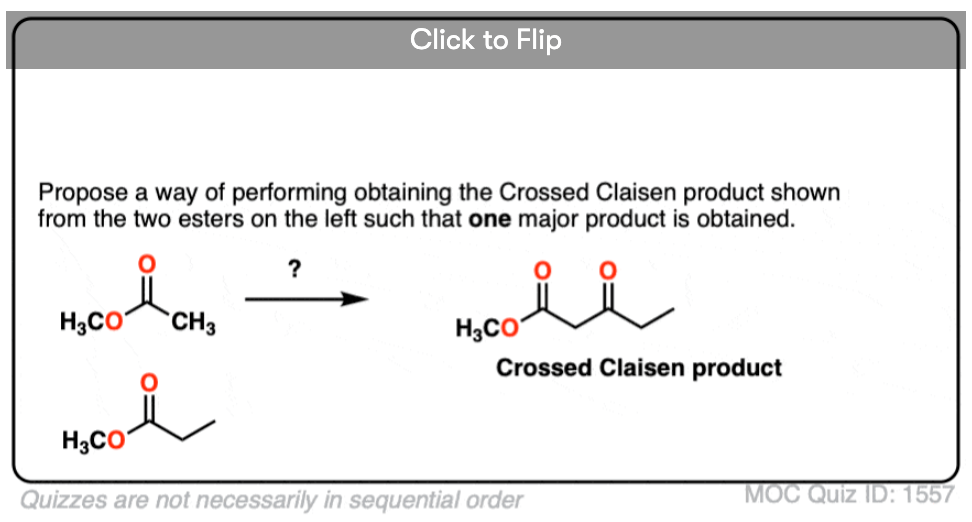
Become a MOC member to see the clickable quiz with answers on the back.
5. Why does the Claisen require a full equivalent of base, but the aldol is catalytic in base?
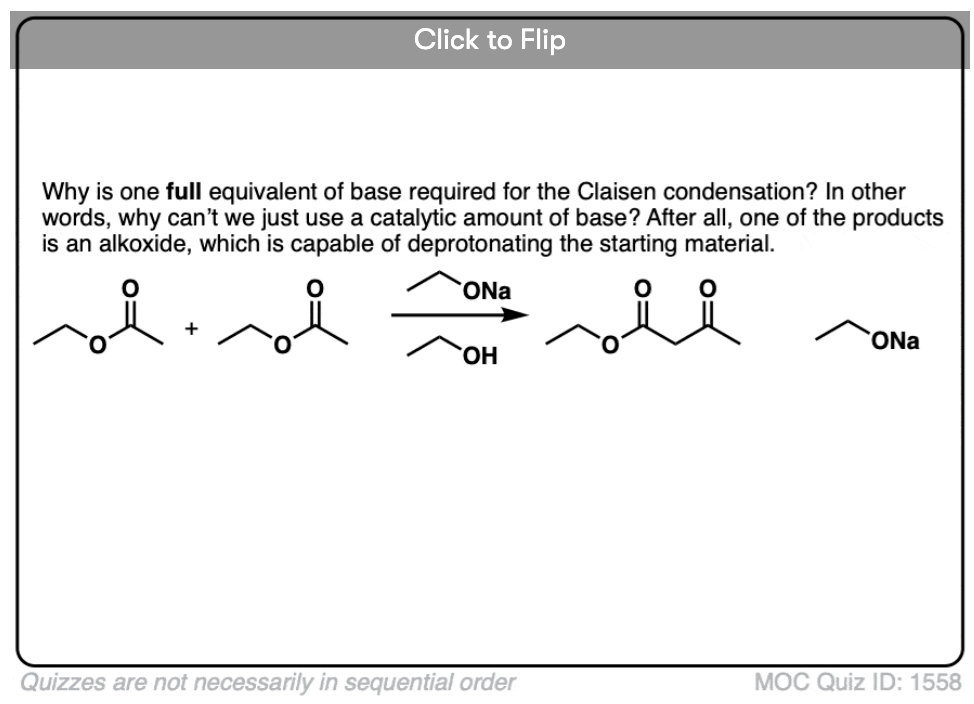
Become a MOC member to see the clickable quiz with answers on the back.
6. A chemist was trying to form the following beta-keto ester through a Dieckmann condensation but was unsuccessful. Why doesn’t this Dieckmann condensation occur?
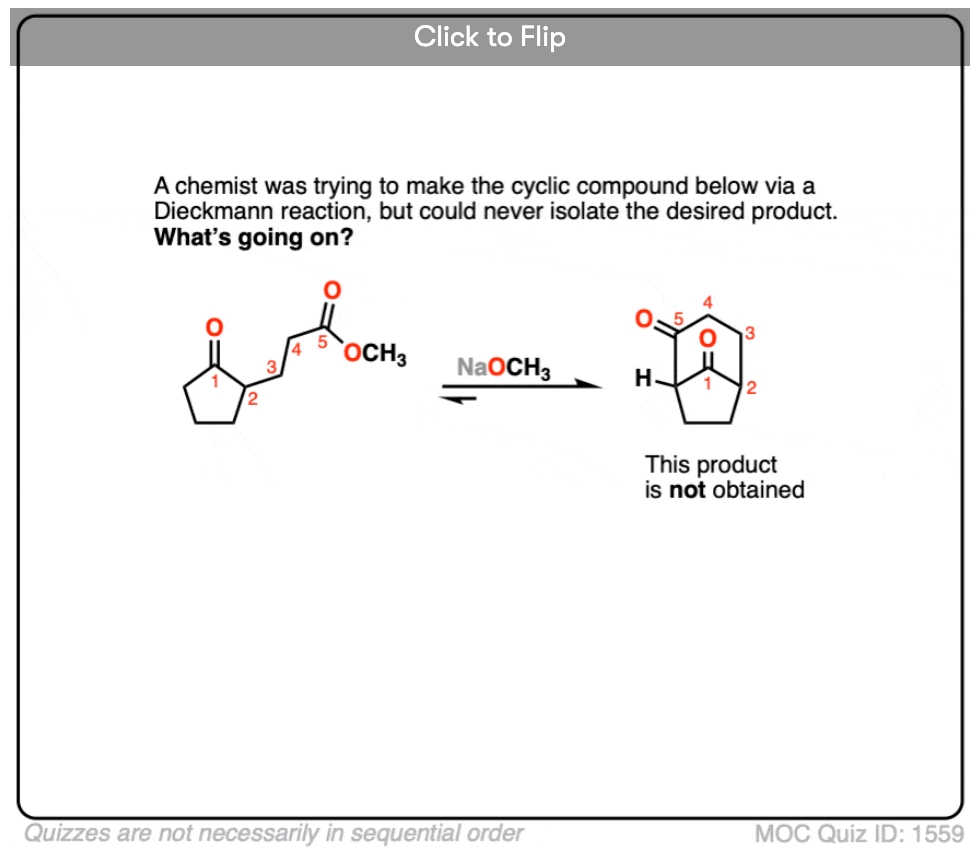
Become a MOC member to see the clickable quiz with answers on the back.
(Advanced) References and Further Reading
The Claisen condensation is also known as the Claisen-Geuther reaction after the first people to publish on this transformation, Ludwig Claisen and A. Geuther.
- Untersuchungen über die einbasischen Säuren
A. Geuther
Arch. Pharm. 1863, 166 (2), 97-110
DOI: 10.1002/ardp.18631660202
The original report of the reaction that came to be known as the Claisen condensation. Formation of ethyl acetoacetate from ethyl acetate and base. - Ueber die Einführung von Säureradicalen in Ketone
L. Claisen
Chem. Ber. 1887, 20 (1), 655-657
DOI: 10.1002/cber.188702001150
This is often referenced as Claisen’s first work on this reaction, although it appears to be a reaction between a ketone enolate and an ester, not between an ester enolate and an ester. See: https://zenodo.org/record/1425461#.X1EeLRNKh25
- The Acetoacetic Ester Condensation and Certain Related Reactions
Hauser, Charles R.; Hudson, Jr., Boyd E.
Org. React. 1942, 1, 266-302
DOI: 10.1002/0471264180.or001.09
Organic Reactions, published and maintained by the ACS division of Organic Chemistry, is a source of comprehensive reviews on various transformations in organic chemistry. This particular review is from the very first issue, in 1942, and covers all instances of the Claisen condensation known up until that time. Detailed experimental procedures are provided towards the end.The Dieckmann condensation is the intramolecular variant of the Claisen condensation. - Zur Kenntniss der Ringbildung aus Kohlenstoffketten
W. Dieckmann
Chem. Ber. 1894 27 (1), 102-103
DOI: 10.1002/cber.18940270126
First report by W. Dieckmann on the intramolecular variant of this reaction. - Ueber cyklische β‐Ketoncarbonsäureester
W. Dieckmann
Just. Lieb. Ann. Chem. 1901, 317 (1), 27-109
DOI: 10.1002/jlac.19013170104 - Claisen Condensation as a Facile Route to an α-Alkoxy-cinnamate: Synthesis of Ethyl (2S)-2-Ethoxy-3-(4-hydroxyphenyl)propanoate
Mats T. Linderberg, Mikael Moge, and Sivaprasad Sivadasan
Organic Process Research & Development 2004, 8 (6), 838-845
DOI: 1021/op040006z
Organic Process Research & Development (‘OPRD’) is also a great place to look for robust organic transformations, since reactions need to be clean, high-yielding, and not require extremely high or low temperatures when scaled up. - Acylation of Esters, Ketones and Nitriles
Brian R. Davis, Peter J. Garratt
Synthesis 1991, 2, 795-863
DOI: 10.1016/B978-0-08-052349-1.00050-0
This review covers both the Claisen and Dieckmann condensations, as well as other related chemistry. - Enantiospecific Synthesis of Carbapentostatins
Jonathan Z. Ho, Rafat M. Mohareb, Jin Hee Ahn, Tae Bo Sim, and Henry Rapoport
The Journal of Organic Chemistry 2003, 68 (1), 109-114
DOI: 10.1021/jo020612x
The Dieckmann condensation also has utility in modern organic synthesis, as it can be used as a annulation (ring-forming) reaction, as can be seen in this total synthesis by Prof. Henry Rapoport (UC Berkeley).
Hi! Quick question, if the ester is treated with a strong base wouldn’t the the reaction be a saponification instead?
Hi, if the ester is treated with hydroxide ion HO(-) that will convert it into a carboxylic acid (and then a carboxylate salt) which is a saponification.
If the ester is treated with an alkoxide base RO(-) then one thing that could happen is not saponification, but *transesterificaation* which is a nucleophilic acyl substitution reaction. E.g. CH3COOCH3 plus NaOCH2CH3 could give CH3COOCH2CH3 plus NaOCH3.
To avoid transesterification in the Claisen we usually make the added base the same as whatever is attached to the ester.
I don’t get it- why doesn’t RO- just attack carbon in c=o for nucleophilic addition reaction in the first step?
It can, but it’s reversible. Like driving down a dead-end street, eventually it will reverse course and go somewhere else.
Will I have access to pdf guides if i pay for monthly course?
No, the pdf guides are one-time downloads, whereas the monthly subscription is for the reaction guide and the quizzes, which are probably too big to download.