Enols and Enolates
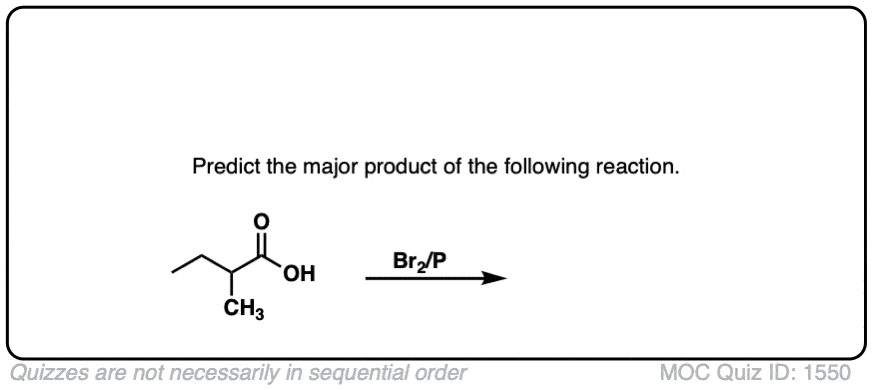
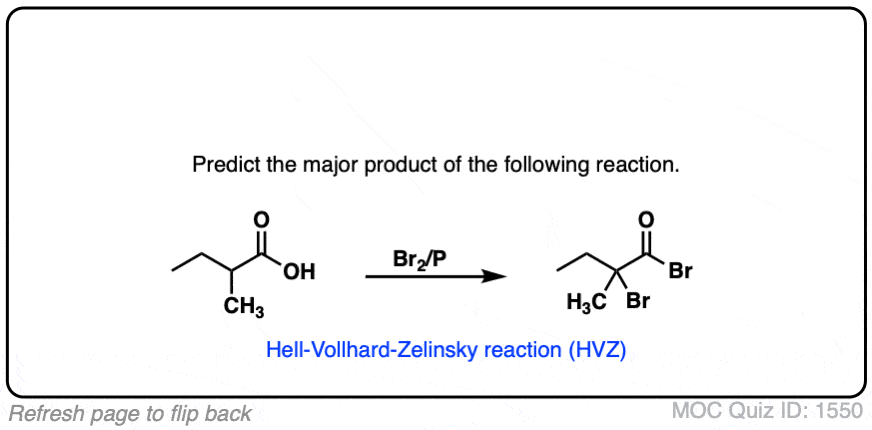
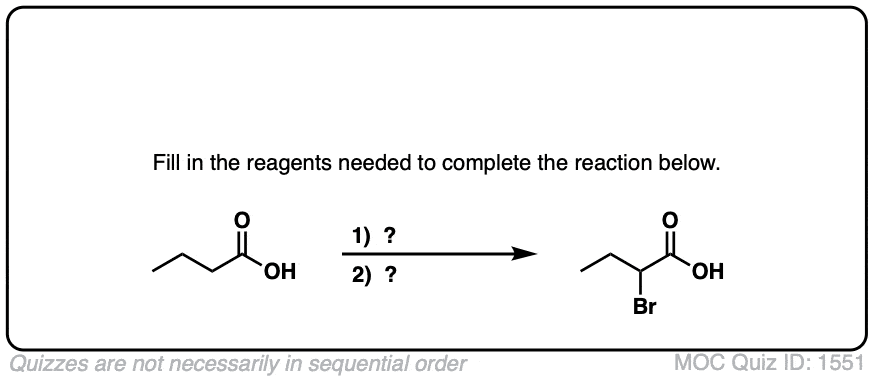
The Hell–Volhard–Zelinsky Reaction
Last updated: November 29th, 2022 |
A Guided Tour Through “Hell” : The Hell Volhard Zelinsky (HVZ) Reaction
- The Hell-Volhard-Zelinsky reaction of carboxylic acids results in an alpha bromo carboxylic acid.The reaction proceeds in 4 steps:
- 1) substitution of OH for Br to give an acyl bromide,
- 2) keto-enol tautomerism,
- 3) bromination of the enol, and
- 4) hydrolysis of the acid bromide to give the carboxylic acid.
- The resulting alpha-bromo acids can then be transformed into alpha-amino acids.
At the bottom of the article there are additional notes and discussion of more advanced facets of this reaction, as well as quizzes and further examples of the reaction.
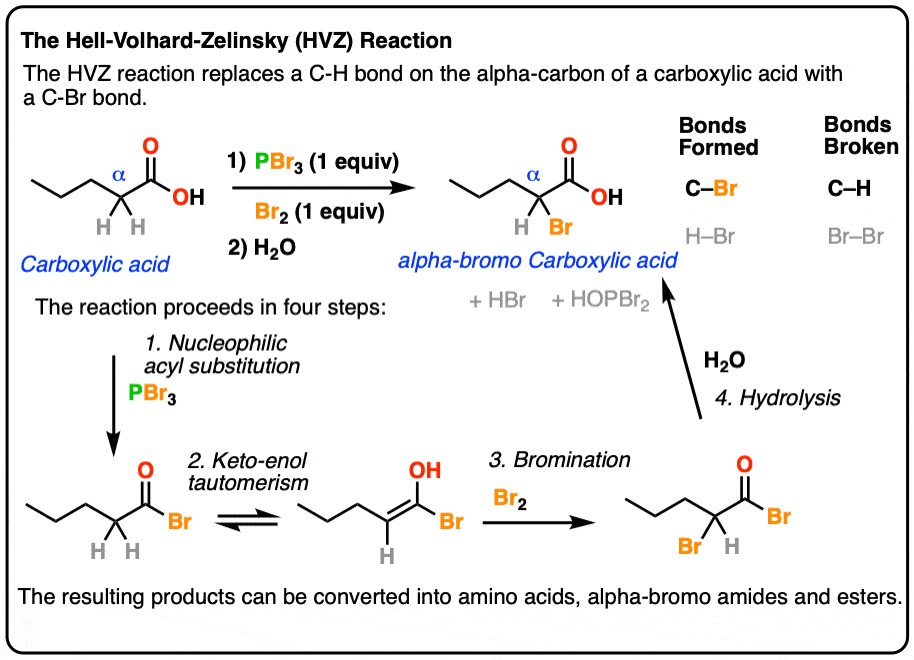
Table of Contents
- The Hell-Volhard-Zelinsky Reaction
- Red Phosphorus (P) And Bromine: The Reactants From “Hell”
- Descent Into Hell: PBr3
- Highway To Hell: Enolization
- Hell’s Kitchen: Bromination Of The Enol
- Hellish Waters: Hydrolysis Of The Acid Bromide
- Summary of the Hell-Volhard Zelinsky Reaction
- Notes
- [Supplemental]: Synthesis of Amino Acids
- [Supplemental]: A Kinder, Gentler Road Through Hell
- Quiz Yourself!
- (Advanced) References and Further Reading
1. The Hell-Volhard-Zelinsky Reaction
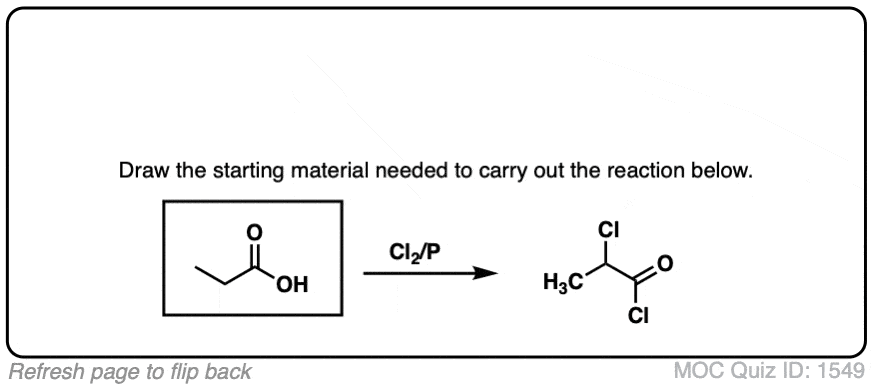
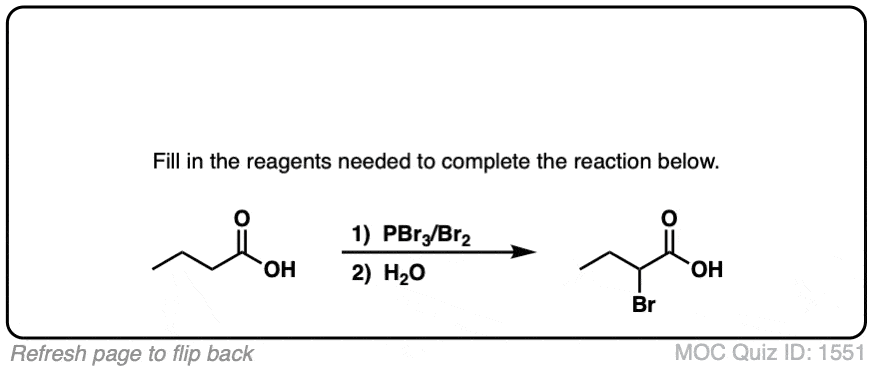
If you take a carboxylic acid like the one below, and treat it with PBr3 and excess Br2, then add water, you get an alpha-bromo acid. Like this:
At first glance, it looks like a simple substitution reaction, since all we’re doing is breaking C-H and forming C-Br on the same carbon. Simple, right?
Well… not exactly.
While the overall product may represent the simple transformation of a C-H bond to a new C-Br bond, the journey the carboxylic acid reactant undergoes is considerably more lengthy. Hellish, even.
You know those Rube Goldberg machines that require 15 intricate steps just to wipe a napkin? It’s kind of like that.
As we’ll see, there are 4 stages to the HVZ. None of the individual steps are new in and of themselves, but it can be a lot to keep track of. The four stages are:
- Conversion of the carboxylic acid to the acyl bromide
- Tautomerization of the acyl bromide to its enol form (breaking C-H)
- Bromination of the alpha-carbon of the enol (forming C-Br)
- Hydrolysis of the acid bromide to the carboxylic acid.
It’s a bit like a huge math equation where almost all of the terms cancel out at the end except for the breakage of C-H and the formation of C-Br.
For this infernal contraption you can thank one Carl Magnus von Hell, who first reported a version of this reaction in 1881. Contributions to the scope and understanding of the reaction by two subsequent researchers led to the shackling of Hell’s name together with Volhard and Zelinsky in a process we now call the Hell-Volhard-Zelinsky reaction. Following the modern practice of abbreviating three-component names by their initials, it is often just called the HVZ reaction for short, or “the Hell” for fun.
Why is it important? Well, there are some interesting applications. The products of the Hell can be used to make amino acids, for example, [see reference below] and not only that, the final acid bromide product can be quenched with other nucleophiles besides water to give alpha-halo esters and alpha halo amides if desired.
2. Red Phosphorus (P) And Bromine: The Reactants From “Hell”
First, let’s clear one thing up. Although in the first example the reagents were written PBr3 and Br2, it is not uncommon to see the reagents for this reaction written like P + Br2 . What does this mean?
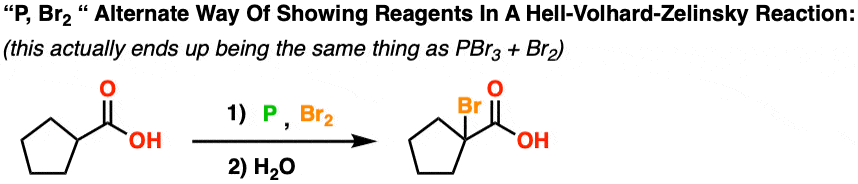
P/Br2 really just means the same as PBr3 / Br2. Let me explain.
The P here stands for elemental phosphorus. Like elemental carbon, which can present itself as graphite, diamond, or buckminsterfullerene, elemental phosphorus forms several “allotropes“. The P in use here is red phosphorus and is reasonably air-stable unless provoked, such as when it is dragged across a rough surface. This makes it useful for applications like match heads, where you’ve probably encountered it at some point. (Another allotrope of phosphorus, white phosphorus, needs no provocation to spontaneously combust in air and is often used in munitions. Pro tip: This is a reagent more suited to a DMZ than the HVZ : – ). )
Bromine (Br2) likely needs no introduction, as it is familiar to most from its reactions with alkenes. But if you haven’t had the “pleasure” of working with bromine in person, here’s a description from a previous article:
Br2 fumes like a bastard. Once you open the bottle, orange vapors start spewing everywhere, and if you haven’t put the bottle deep into the fume hood, you will soon be savoring the unforgettable aroma of Br2 (mixed with HBr) in your nostrils. It’s extremely dense (d=3.19) with a high vapor pressure and therefore drops of it tend to fall from whatever you’re using to dispense it with, Jackson Pollock style, leaving a little trail of violently fuming orange puddles behind….
The name “bromine” comes from the Greek Bromos for stench, after all. [Periodic videos on Youtube]
Red phosphorus is combines with elemental bromine (Br2) to give phosphorus tribromide (PBr3). [see ref below] To an even greater extent than hipster beverage PBR, PBr3 is nasty. When left out in the air, it will readily combine with any water vapor present to form a new P-O bond along with HBr. [Note 3]
The strength of the P-O bond [about 140 kcal/mol] makes PBr3 very useful for swapping out C-O bonds for C-Br bonds. We’ve seen PBr3 used previously in the conversion of R-OH to R-Br. [See post: PBr3 and SOCl2] In the HVZ, the PBr3 that forms will convert carboxylic acids to acyl bromides.
However, PBr3 by itself is not sufficient to perform the HVZ. That’s why Br2 is added in molar excess of the amount necessary to form PBr3, leaving at least one extra equivalent of Br2 to perform the halogenation of the alpha-carbon.
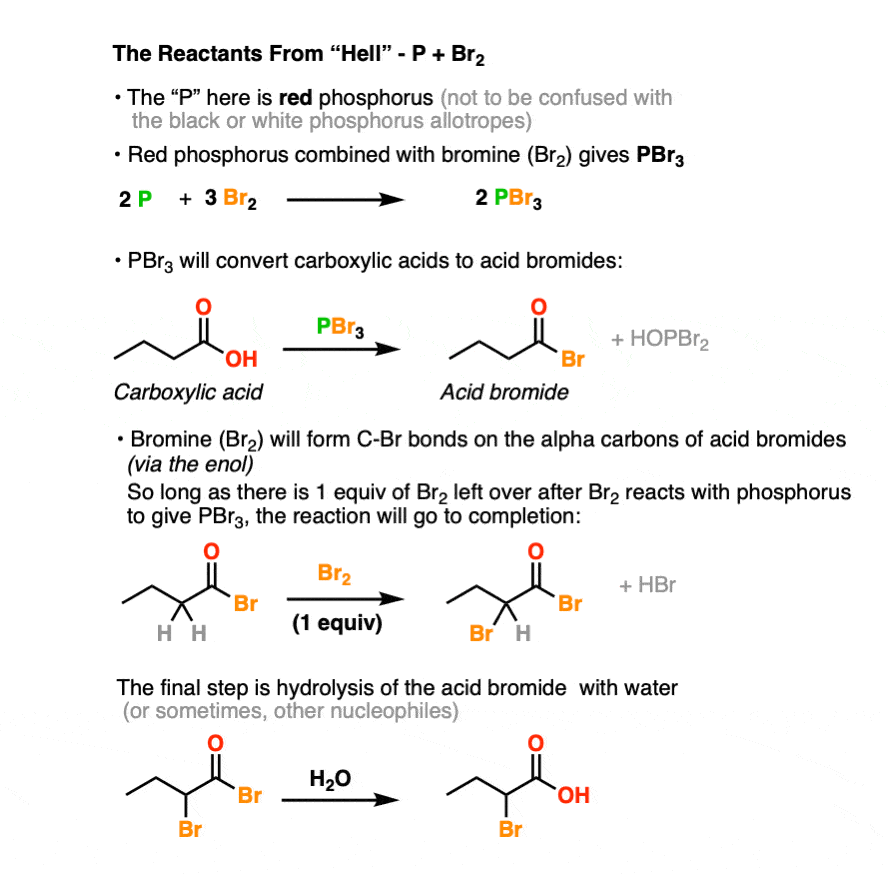
The final step, common to both, is to quench the reaction with H2O, giving the carboxylic acid.
[Note 1 – to keep things simple we are going to follow the introductory textbooks and assume 1 equivalent of PBr3]
3. A Guided Tour Through Hell. Stage 1: PBr3
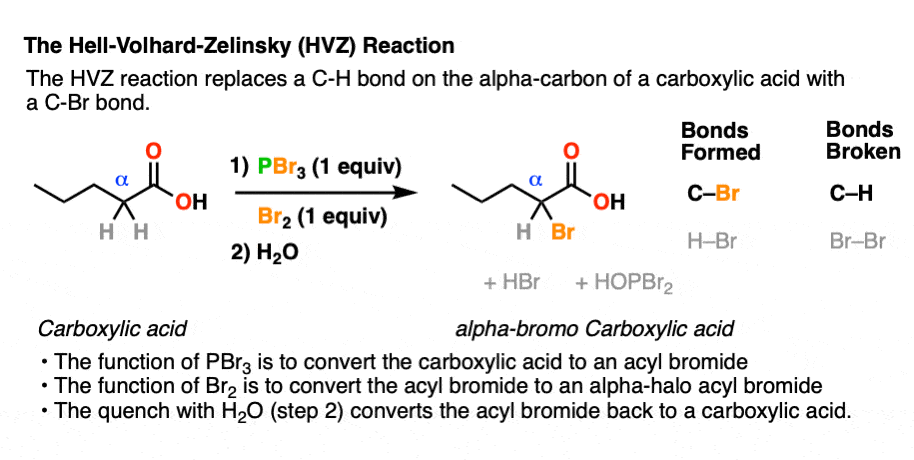
With that out of the way, it’s time take a guided tour through the Hell! This brings us to the first step of the Hell-Volhard Zelinsky reaction: conversion of the carboxylic acid to an acyl bromide.
Since a C-OH bond is being swapped for a C-Br bond this is a technically a substitution reaction. More specifically, this is a nucleophilic acyl substitution reaction since the substitution occurs on an acyl (RC(O)- ) carbon.
The purpose of PBr3 here is to convert the -OH of the carboxylic acid into a good leaving group, with the driving force being formation of the strong P-O bond.
In the first step, an oxygen from the carboxylic acid attacks PBr3, with liberation of Br(-). The bromide ion Br(-), being a good nucleophile, then attacks the carbonyl carbon, forming C-Br and breaking C-O (pi). [This is nucleophilic addition]. The next step is elimination (or “1,2-elimination”) which re-forms C-O (pi) and breaks C-O, liberating HO-PBr2. The result is an acyl bromide.
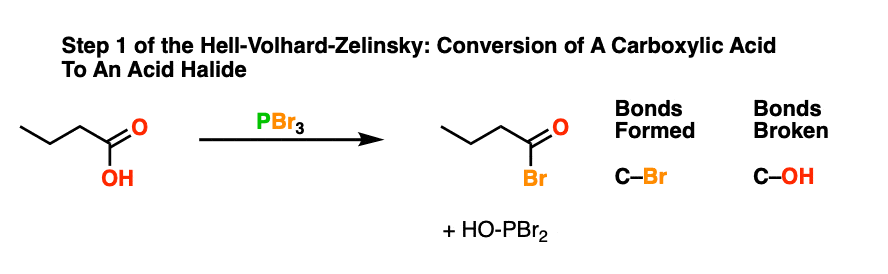
To see the full mechanism, hover here and an image will pop up or click the link.
4. Highway To Hell: Enolization
Recall that the carbon adjacent to a carbonyl carbon is called the “alpha” carbon. All carbonyl compounds with alpha carbons possessing a C-H bond are capable of forming an enol, a type of constitutional isomer more often referred to as a tautomer. Tautomers are constitutional isomers that interconvert.
The acid bromide is in equilibrium with its enol form. The equilibrium lies far to the side of the acid bromide, but the enol form is still accessible. In practice, a single drop of strong acid will help to facilitate the rate of keto-enol interconversion. [Section 3 of a different post: How is Keto-Enol Tautomerism Catalyzed By Acid]
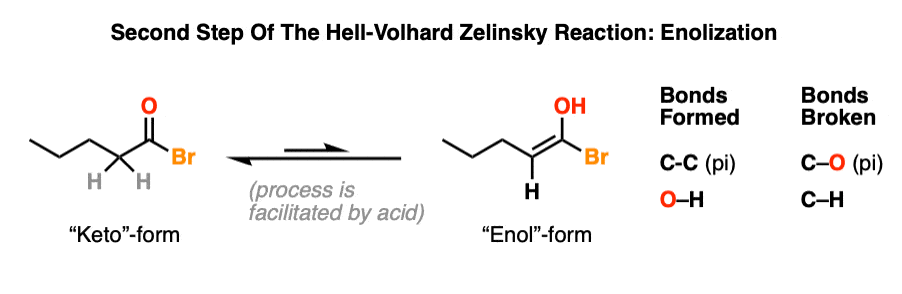
This is the step where the C-H bond on the alpha carbon breaks.
To see the mechanism for enolization, hover here and an image will pop up or click the link.
Note 2: The enol form is much more accessible for the acid bromide than it is for the carboxylic acid, which helps to explain why carboxylic acids do not undergo alpha-bromination directly.
5. Hell’s Kitchen: Bromination Of The Enol
If you recall that the key resonance form of an enol has a negative charge on carbon, then it will not surprise you that enols are nucleophiles.
Enols will readily combine with bromine, ( Br2 ) resulting in formation of a new C-Br bond on the alpha carbon:
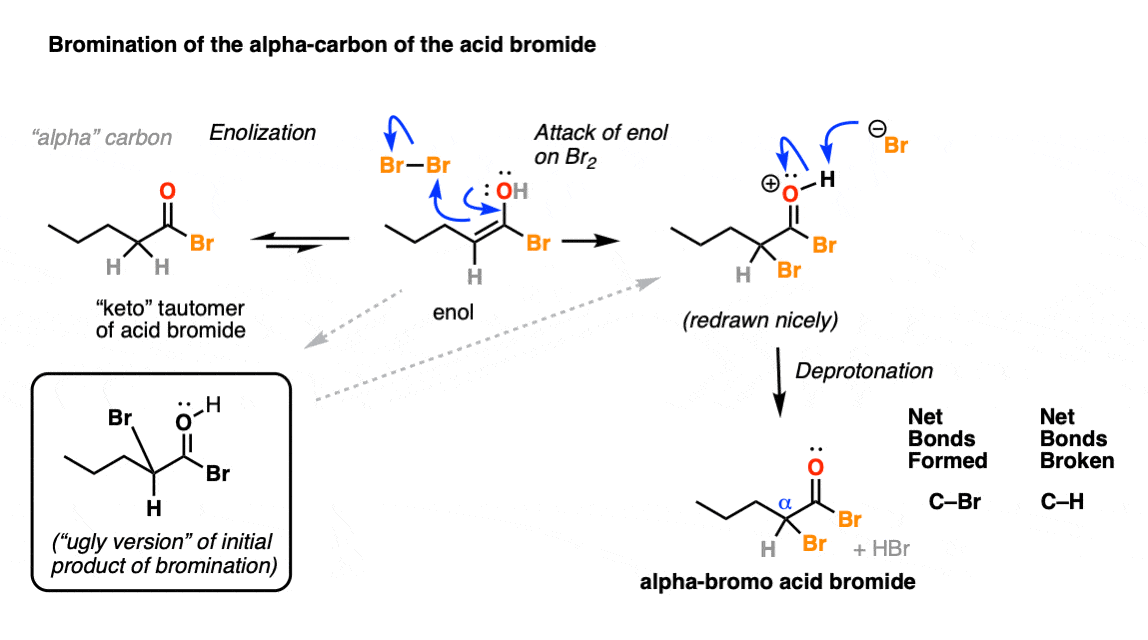
When drawing out the products of these reactions, it can sometimes be helpful to draw the “ugly version” of it first, just showing formation of the new C-Br bond without regard for drawing proper molecular geometry. Once you’ve really nailed down what bonds form and break here, you can then redraw the “ugly” version to a molecule that better shows the tetrahedral geometry of carbon. [See post: How NOT to draw tetrahedral carbon]
6. Hellish Waters: Hydrolysis Of The Acid Bromide
The final step of the HVZ is hydrolysis of the acid bromide to the carboxylic acid.
For our purposes, this generally occurs through addition of water once all of the starting carboxylic acid has been consumed. This “quench” or “workup” step results in breakage of the C-Br bond and formation of the C-O bond.
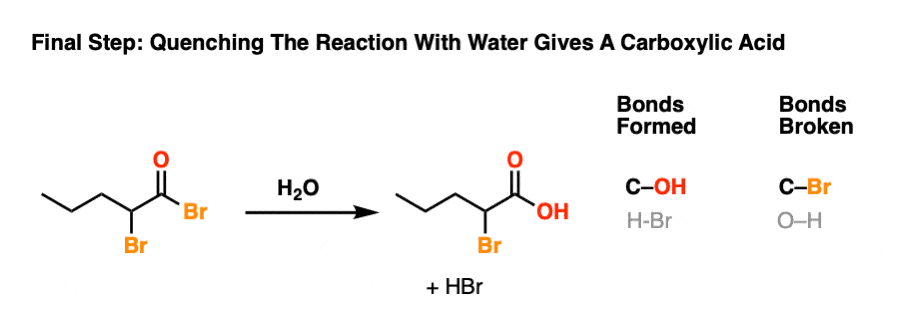
This is another example of nucleophilic acyl substitution. Attack of nucleophile (addition), elimination of leaving group (loss of Br) followed by deprotonation to re-generate the neutral carboxylic acid.
To see the full mechanism, hover here and an image will pop up or click the link.
To spice things up one can also quench with an amine to give an amide or an alcohol to give an ester.
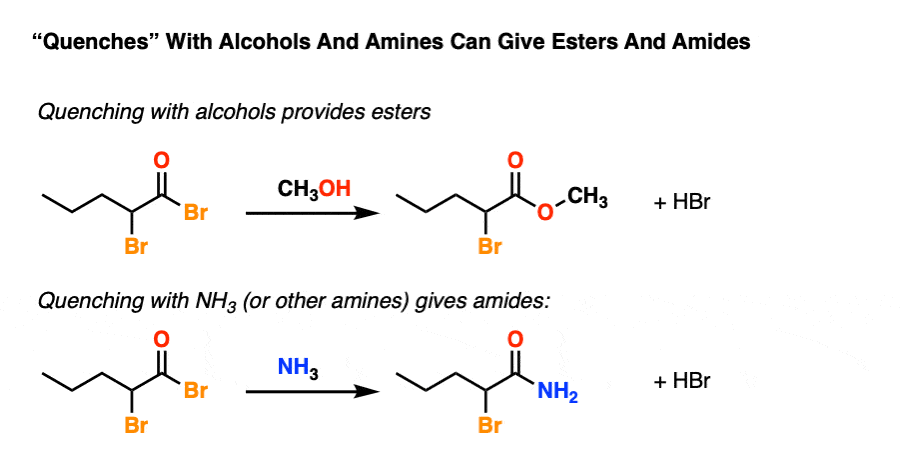
And that pretty much does it.
7. Summary of the Hell-Volhard Zelinsky Reaction
So to summarize, in the HVZ, we’ve seen that a lot of atomic furniture needs to get rearranged just to swap out a C-H bond for a C-Br bond.
- First, the carboxylic acid is converted to the acid bromide (nucleophilic acyl substitution);
- Second, the enol of the acid bromide is formed (keto-enol tautomerism);
- Third, the enol undergoes bromination to give the alpha-bromo acid bromide (enol acting as nucleophile);
- Fourth, the reaction is “quenched” with water to give back an alpha bromo carboxylic acid (another nucleophilic acyl substitution).
None of these steps are new, in and of themselves!
But like a song built out of a combination of familiar chords, they do come together in a sequence that is uniquely… Hellish.
Notes
Note 1. In practice, only a catalytic amount of PBr3 is necessary [see here for example], although this is generally not covered in introductory courses, and it also requires a minor modification to the mechanism that we will not go into in detail here. However, certain parts of the catalytic mechanism *is* good material for exam questions / quizzes (below).
Note 2. Carboxylic acids, by themselves, do not undergo bromination as quickly as acid bromides since their enol forms are less accessible, although I have not been able to find good data on keto-enol equilibrium constants for comparison.
Note 3. PBr3, along with its cousins PCl5 and PBr5, gets my vote as the chemicals “most likely to be found in a rusted-out black crumbling Aldrich can on a shelf in the chemical storage room nobody wants to touch”.
Application: Synthesis of Amino Acids
It’s worth asking: what are the alpha-halo acids used for?
Amino acids are useful, right? The HVZ has been used as a method for the synthesis of various amino acids, through displacement of the halogen with ammonia (NH3).
Here is an application where the HVZ is used to synthesize (racemic) leucine:
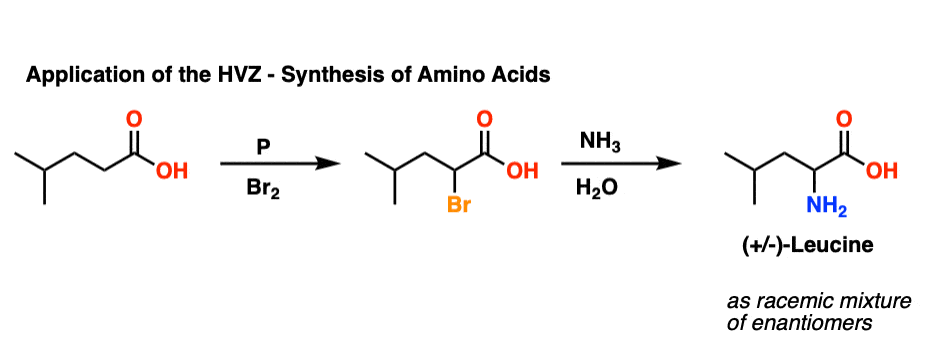
[To be clear, in this reaction NH3 was used as a solution in water, where it is called, “ammonium hydroxide”.]
A Kinder, Gentler Road Through Hell
It has been found [see ref] that acid chlorides are slower to undergo bromination than acid bromides. The cited reason is that acid chlorides are more resistant to enolization than acid bromides. [although to be honest I can’t find a good reference that really supports this with measured equilibrium constants, etc. ]
However it turns out that NBS works really well for the alpha-halogenation of acid chlorides, as found by Gleason and Harpp.
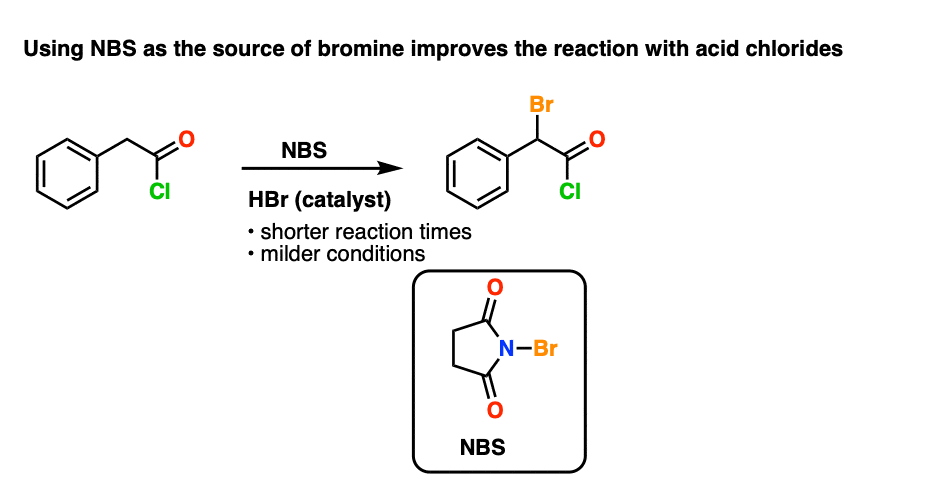
A few drops of strong acid (HBr) promotes the reaction, which is slow otherwise.
As we mentioned above, acids catalyze keto-enol tautomerism; protonating the carbonyl oxygen makes the carbonyl carbon more electrophilic, which in turn increases the acidity of the C-H bond on the alpha carbon. [See post: Acids Promote Keto-Enol Tautomerism]
Besides bringing acid chlorides into the fold (which react slowly under traditional HVZ conditions) NBS is a nice, easy to handle crystalline solid. The reaction also requires significantly milder reaction conditions.
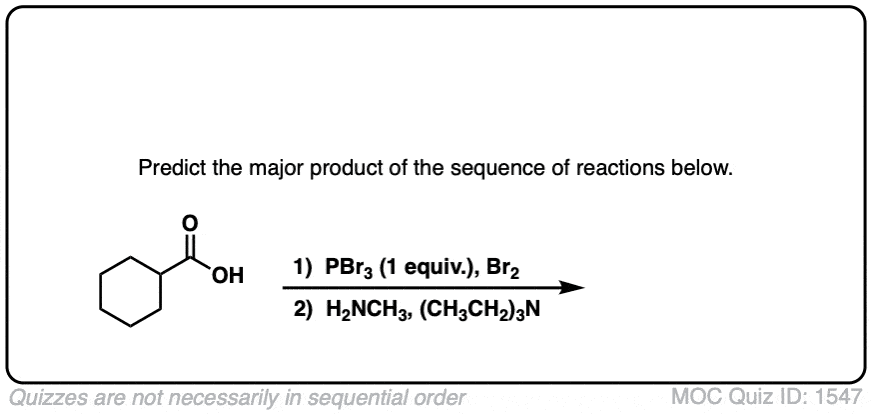
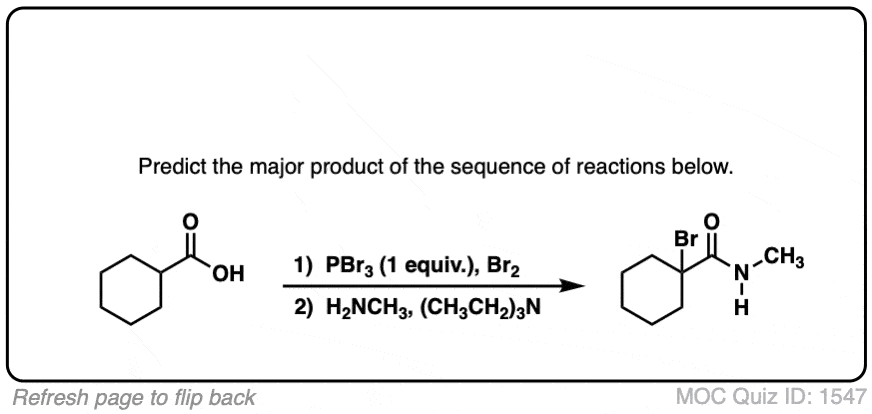
Quiz Yourself!
 Click to Flip
Click to Flip
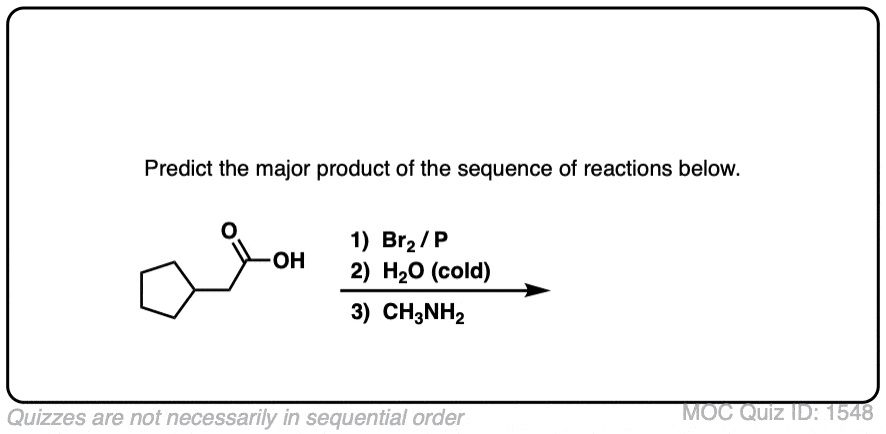 Click to Flip
Click to Flip
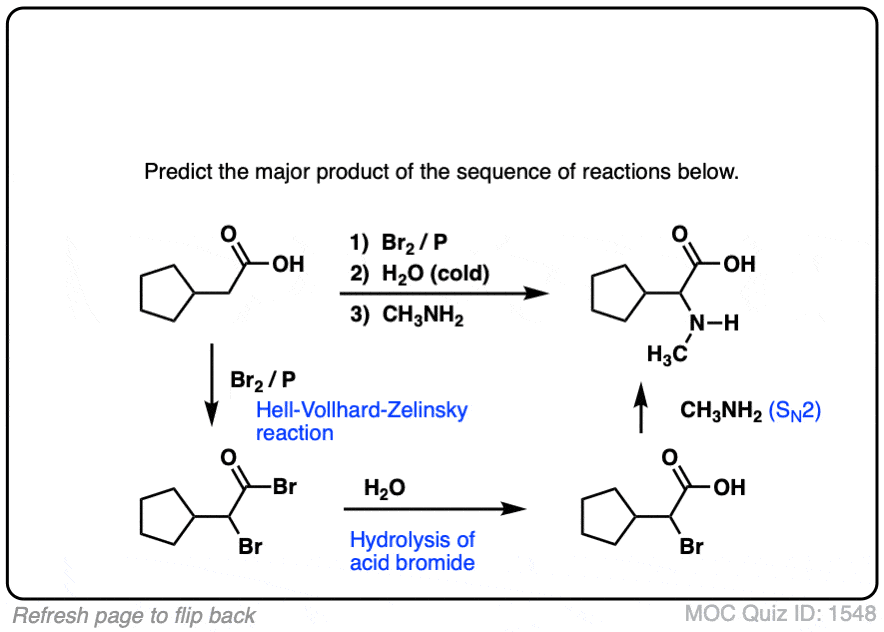
 Click to Flip
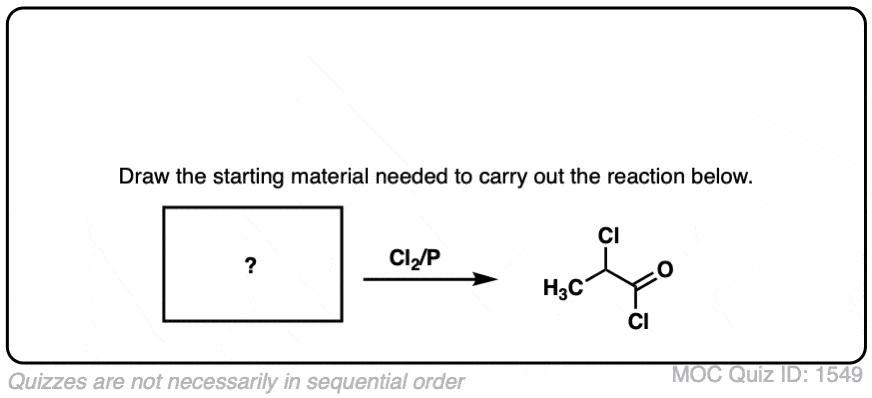
Click to Flip
 Click to Flip
Click to Flip
 Click to Flip
Click to Flip
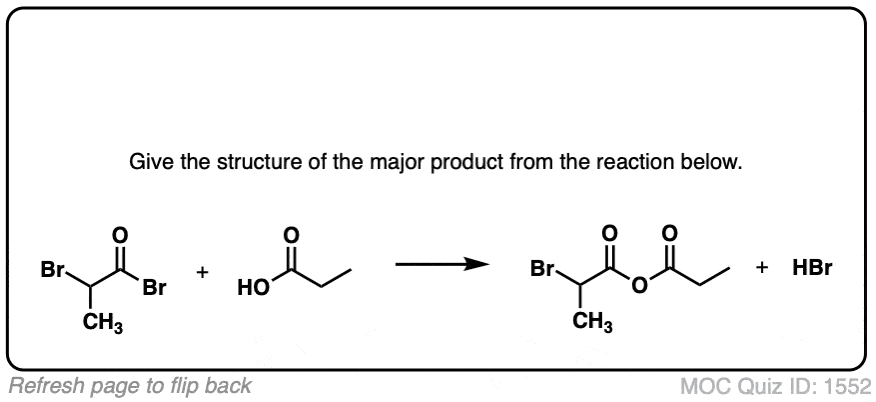
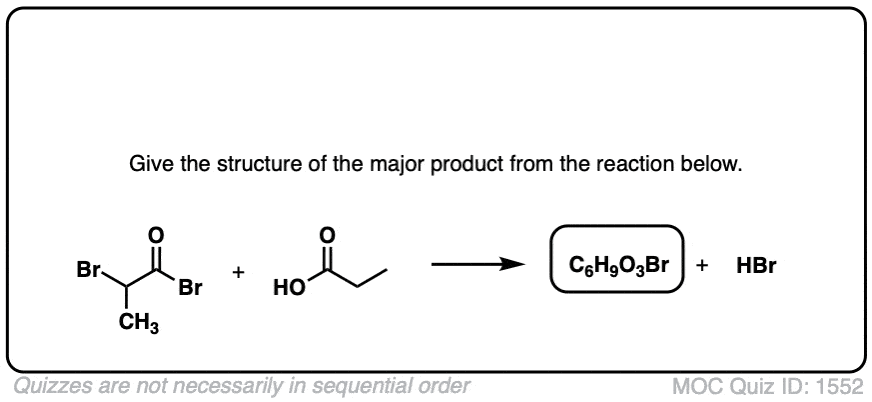 Click to Flip
Click to Flip
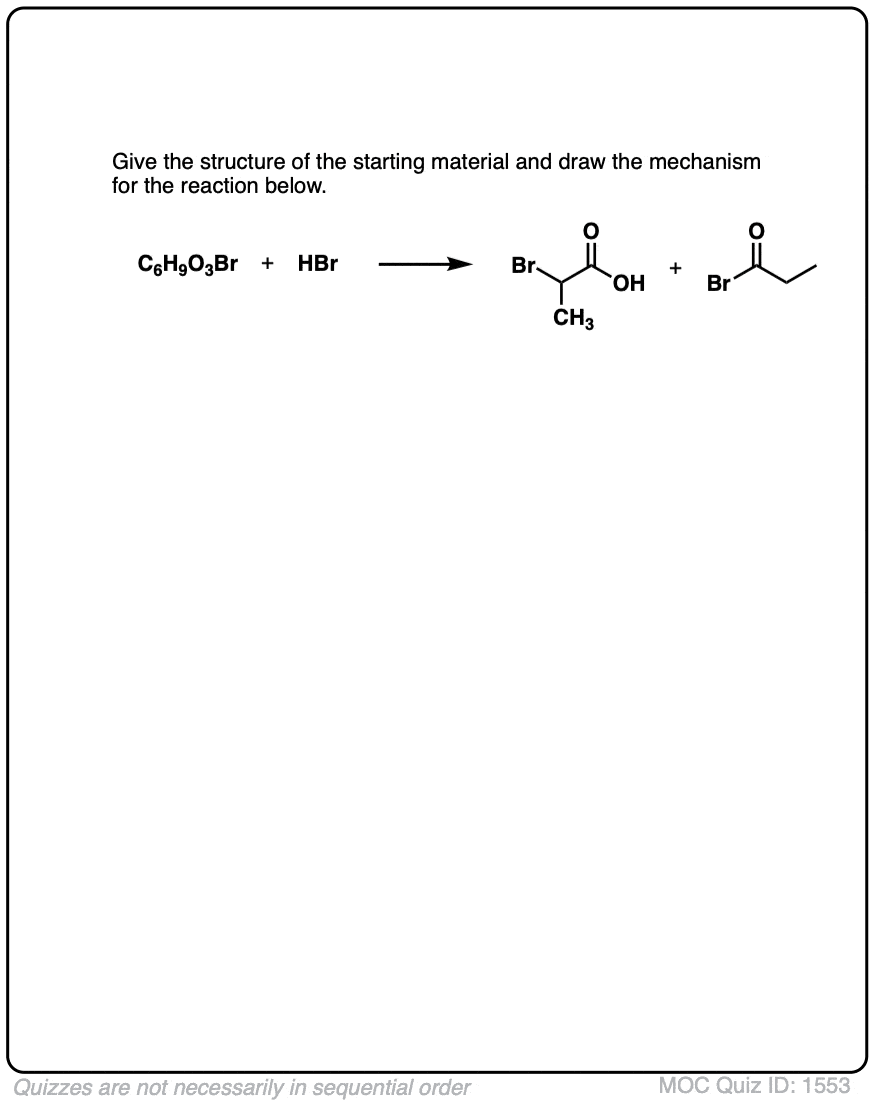 Click to Flip
Click to Flip
(Advanced) References and Further Reading
Some older references here, but still very interesting.
- The Preparation of Acetyl Bromide*
Theodore M. Burton and Ed. F. Degering
Journal of the American Chemical Society 1940 62 (1), 227-227
DOI: 10.1021/ja01858a502
Procedure for the use of PBr3 to make acetyl bromide from acetic acid.
“Phosphorus tribromide can be prepared in 99.5% yield by slow addition of dried bromine into a slight excess of dried red phosphorus…”The original Hell, Vollhard and Zelinsky papers:
- Ueber eine neue Bromirungsmethode organischer Säuren
Carl Magnus von Hell
Ber. 1881, 14 (1), 891-893
DOI: 10.1002/cber.188101401187 - Ueber Darstellung α‐bromirter Säuren
Volhard, J.
Liebig. Ann. Chem. 1887, 242 (1-2), 141-163
DOI: 10.1002/jlac.18872420107 - Ueber eine bequeme Darstellungsweise von α‐Brompropionsäureester
Zelinsky, N.
Ber. 1887, 20 (1), 2026
DOI: 10.1002/cber.188702001452 - The Action Of Halogens On Compounds Containing The Carbonyl Group
Arthur Lapworth.
J. Chem. Soc. 1904, 85, 30-42
DOI: 10.1039/CT9048500030
Important paper proposing enols as intermediates in the halogenation of carbonyl compounds. “The action of bromine on acetone in dilute aqueous solution is exceedingly slow, but becomes more rapid in presence of acids [such as H2SO4, HCl, HNO3, but not so much AcOH]”. The rate of bromination was found to be proportional to the concentration of acetone but not Br2. “It seems probable, then, that the bromination of acetone under the conditions maintained is best regarded as the result of a slow, reversible change effected in the acetone by [H+] followed by an almost instantaneous bromination of the product, a change which is not appreciably reversible. This intermediate product is perhaps the enolic form of the ketone.” - The Reaction of Bromine With Aliphatic Acids. Part II. The Relative Speeds of Bromination Of Acetyl Bromide and Acetyl Chloride
Watson, H. B.
J. Chem. Soc. 1928, 1137.
DOI: 10.1039/JR9280001137
Comparative study on bromination of acid bromides vs acid chlorides. “Bromine reacts with acetyl chloride more slowly than with acetyl bromide.” Proposes reaction proceeds through enol of the acid bromide. - dl-LEUCINE
Carl S. Marvel
Organic Syntheses, 1941, 21, 74.
DOI: 10.15227/orgsyn.021.0074
First step here is a classic HVZ with P and Br2. Subsequently the carbon undergoes substitution to eventually afford racemic leucine.“The method described above is essentially that of Fischer (Ber. , 37, 2486 1904) and is the cheapest and best procedure for the synthesis of large amounts of this amino acid.” - The Kinetics of the Reaction between Bromine and Acetyl Bromide in Nitrobenzene Solution
Carl Cicero and Dan MathewsThe Journal of Physical Chemistry 1964 68 (3), 469-471
DOI: 10.1021/j100785a005
A study on the bromination of acid bromides that supports the enol mechanism.The following two papers describe useful modifications to the Hell-Volhard-Zelinsky reaction using NBS (N-bromo succinimide, which is easier to handle than Br2 or PBr3):
- α-Bromination of acid halides
John G. Gleason, David N. Harpp
Tetrahedron Lett. 1970, 11 (39), 3431-3434
DOI: 10.1016/S0040-4039(01)98495-3 - Characterization of Keto-Enol Tautomerism of Acetyl Derivatives from the Analysis of Energy, Chemical Potential, and Hardness
Patricia Pérez and Alejandro Toro-Labbé
The Journal of Physical Chemistry A 2000 104 (7), 1557-1562
DOI: 10.1021/jp9930797
Calculation for barriers to enolization for acetaldehyde, acetone, acetyl chloride, and acetyl bromide suggests barrier to enolization for the acyl halides is similar (about 25 kcal/mol vs. 13-14 for aldehydes/ketones). Kind of strange in light of Watson’s study. - A simple and efficient method of preparing α-bromo carboxylic acids
Lian Hao Zhang, Jianxin Duan, Yuelian Xu, William R. Dolbier, Jr.
Tetrahedron Lett. 1998 39 (52), 9621-9622
DOI: 10.1016/S0040-4039(98)02204-7
Prof. Dolbier (U. Florida) is well known for his work in fluorine chemistry. - Enzymatic Lactonization Stategy for Enantioselective Synthesis of a Tetrahydrolipstatin Synthon
Sharma, S. Chattopadhyay
The Journal of Organic Chemistry 1999 64 (22), 8059-8062
DOI: 10.1021/jo990370+
The synthesis of 5 in this paper is through a classical Hell-Volhard-Zelinsky reaction (see 6 in the experimental section for a procedure).
Benzoic acid doesnot show hvz as it doesnot have alpha Hydrogen…. You need at least one alpha H for keto enol tautomerization
hi, how to do it with benzoic acid
Won’t work, as there is no alkyl group adjacent to the carbonyl carbon.
Hello !
As with all your articles, thank you again for this level of technical and educational excellence!
However, I must point out that there are a lot of typos between the terms “acid” and “acyl”.