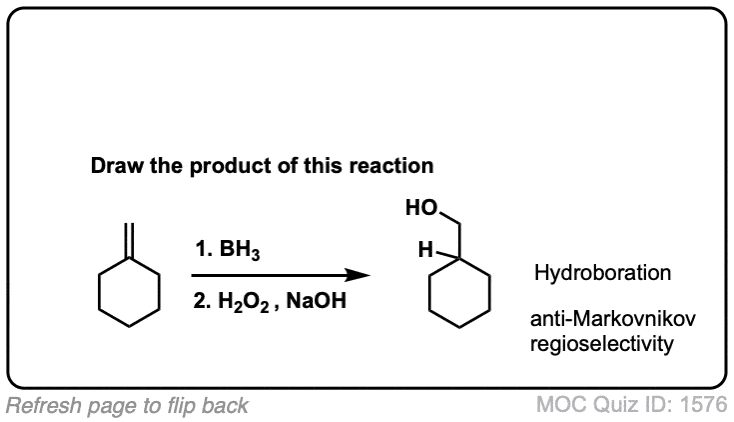
Hydroboration of Alkenes
Description: Hydroboration-oxidation transforms alkenes into alcohols. It performs the net addition of water across an alkene.
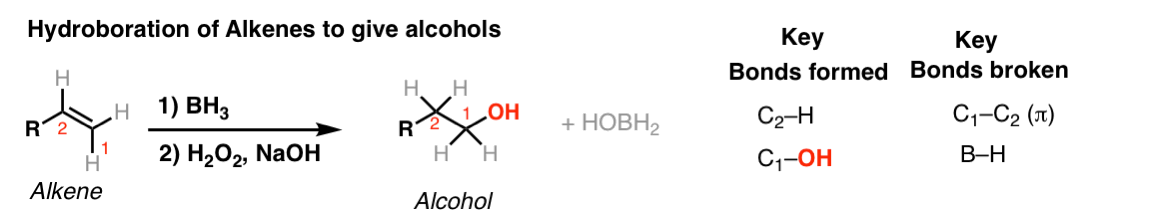
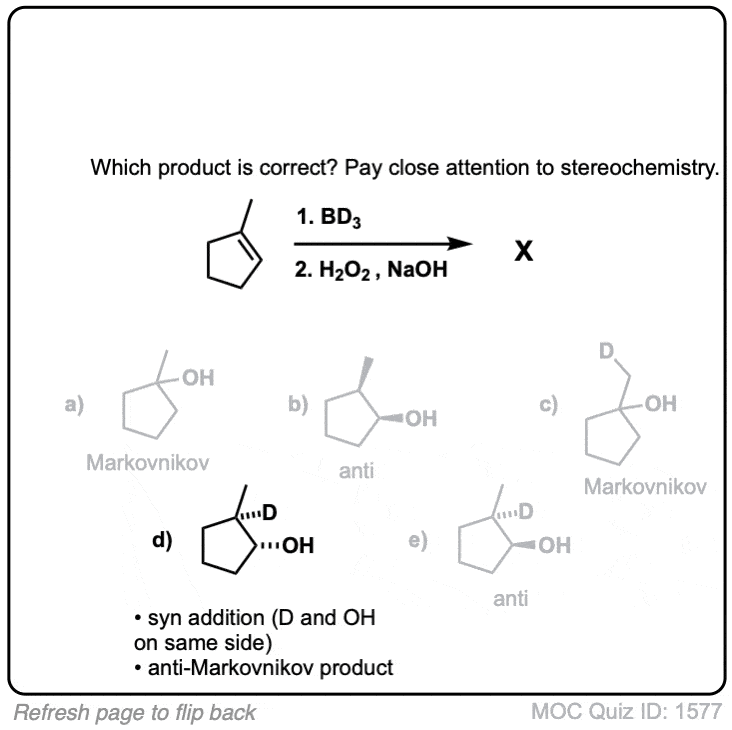
Notes: Note that the oxygen is always attached at the less substituted carbon (anti-Markovnikoff). Furthermore the stereochemistry is always syn (H and OH add to same side of the alkene).
The boron byproduct will depend on the # of equivalents of BH3 used reative to the alkene. Here their molar ratio is 1:1. One equivalent of BH3 can hydroborate up to 3 equivalents of alkene.
BH3-THF is the same as BH3. Tetrahydrofuran (THF) is merely a solvent. Sometimes B2H6 is written, which is another form of BH3. It behaves in exactly the same way as BH3.
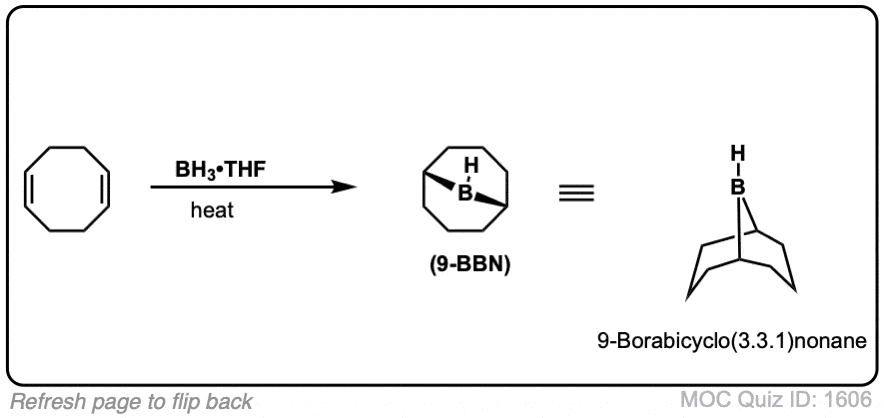
You might also see 9-BBN or (Sia)2BH. These are hydroboration reagents in which two of the H atoms in BH3 have been replaced by carbon atoms. They will do the exact same reaction as BH3.
Examples:
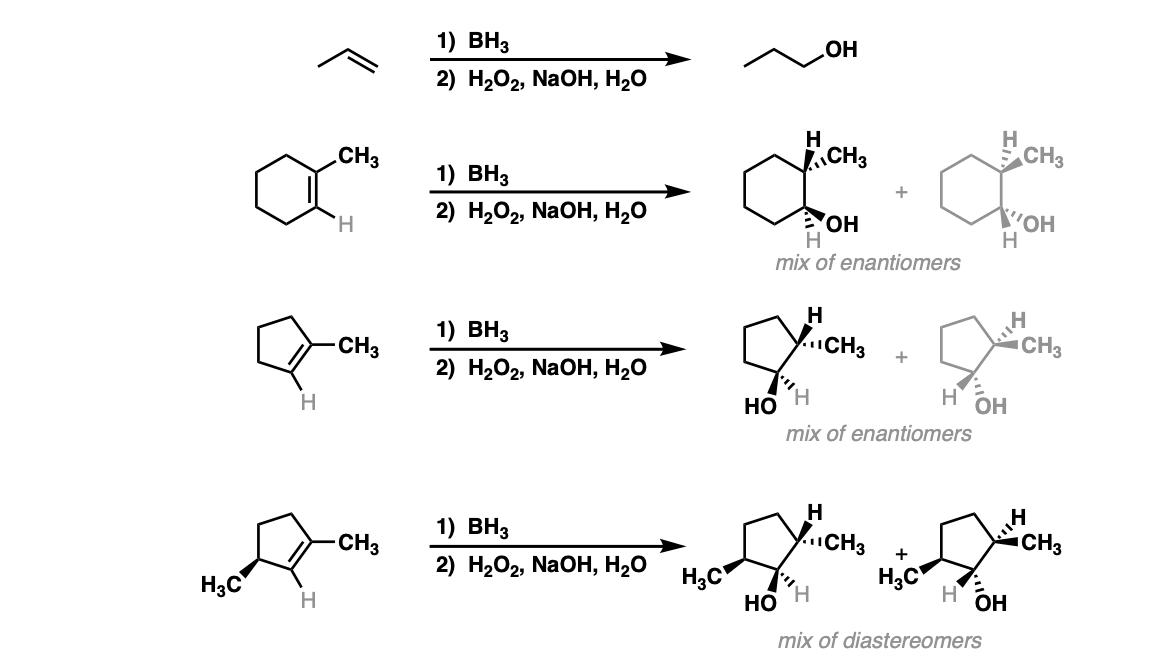
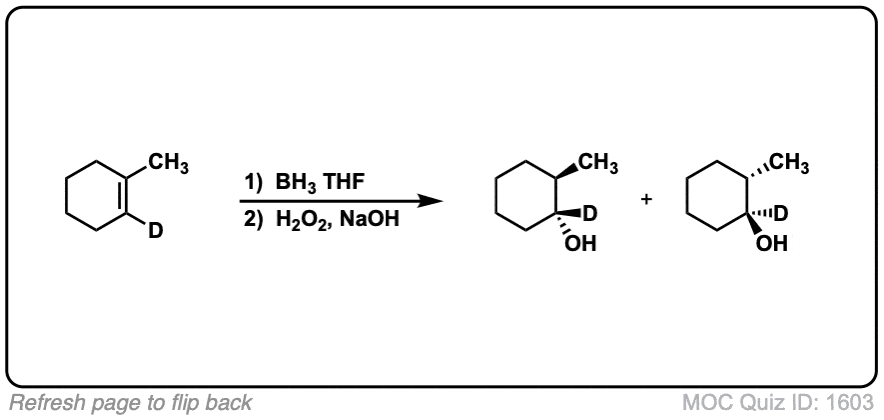
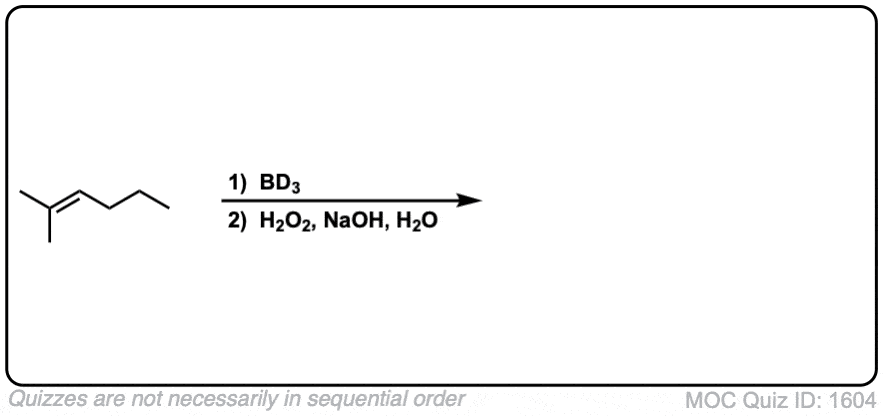
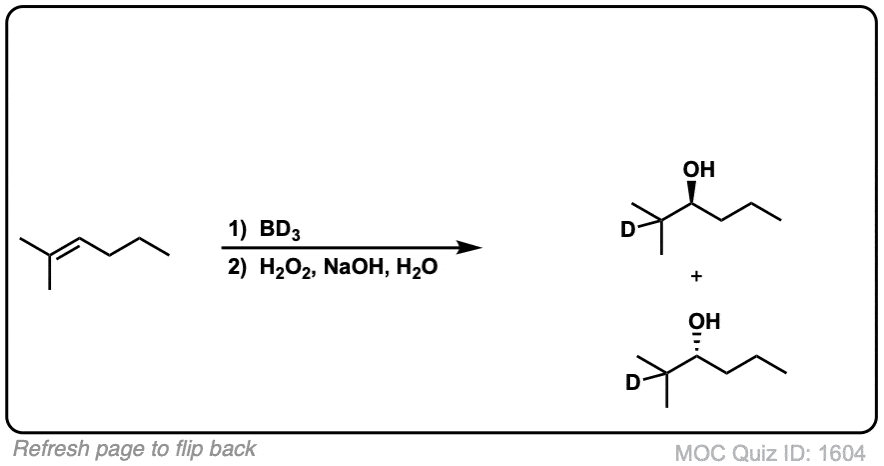
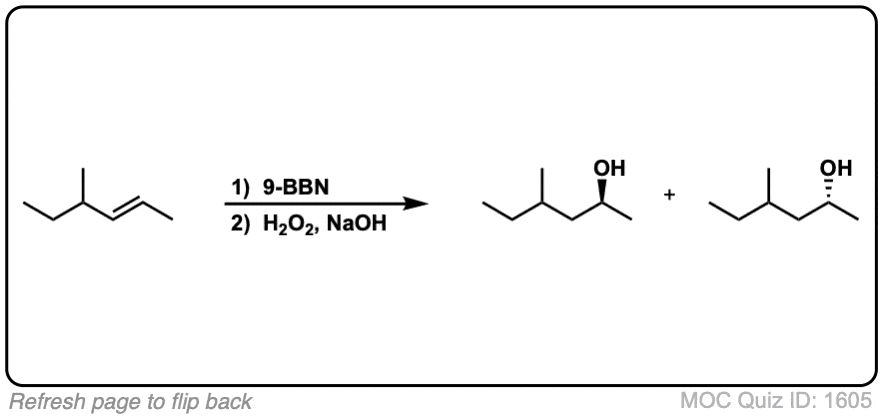
Notes: Example 1 just shows a simple anti-Markovnikov addition of BH3 to an alkene. Examples 2 and 3 show the hydroboration of a cyclic alkene. Note the syn addition; the C-H bond and C-OH bond are formed on the same side of the ring (this results in a mixture of enantiomers in this case). Example 4 has an existing chiral center (the methyl group) which is unaffected by hydroboration, so the result will be a mixture of diastereomers.
Mechanism:
The reaction begins with the concerted syn addition of B and H across the double bond, with the boron adding to the less substituted carbon (Step 1, arrows A and B). In the second step, hydrogen peroxide and a base such as NaOH are added. the NaOH deprotonates the hydrogen peroxide (Step 2, arrows C and D) which makes the conjugate base of hydrogen peroxide (a better nucleophile than H2O2 itself). The resulting NaOOH then attacks the boron (Step 3, arrow E). This sets up the key migration step, where the carbon-boron bond migrates to the oxygen bound to boron, breaking the weak oxygen-oxygen bond (Step 4, arrows F and G). The OH expelled then comes back to form a bond on the boron (Step 5, arrows H and I) resulting in the deprotonated alcohol (alkoxide). The alkoxide is then protonated by water or some other comparably acidic species (Step 6, arrows J and K).
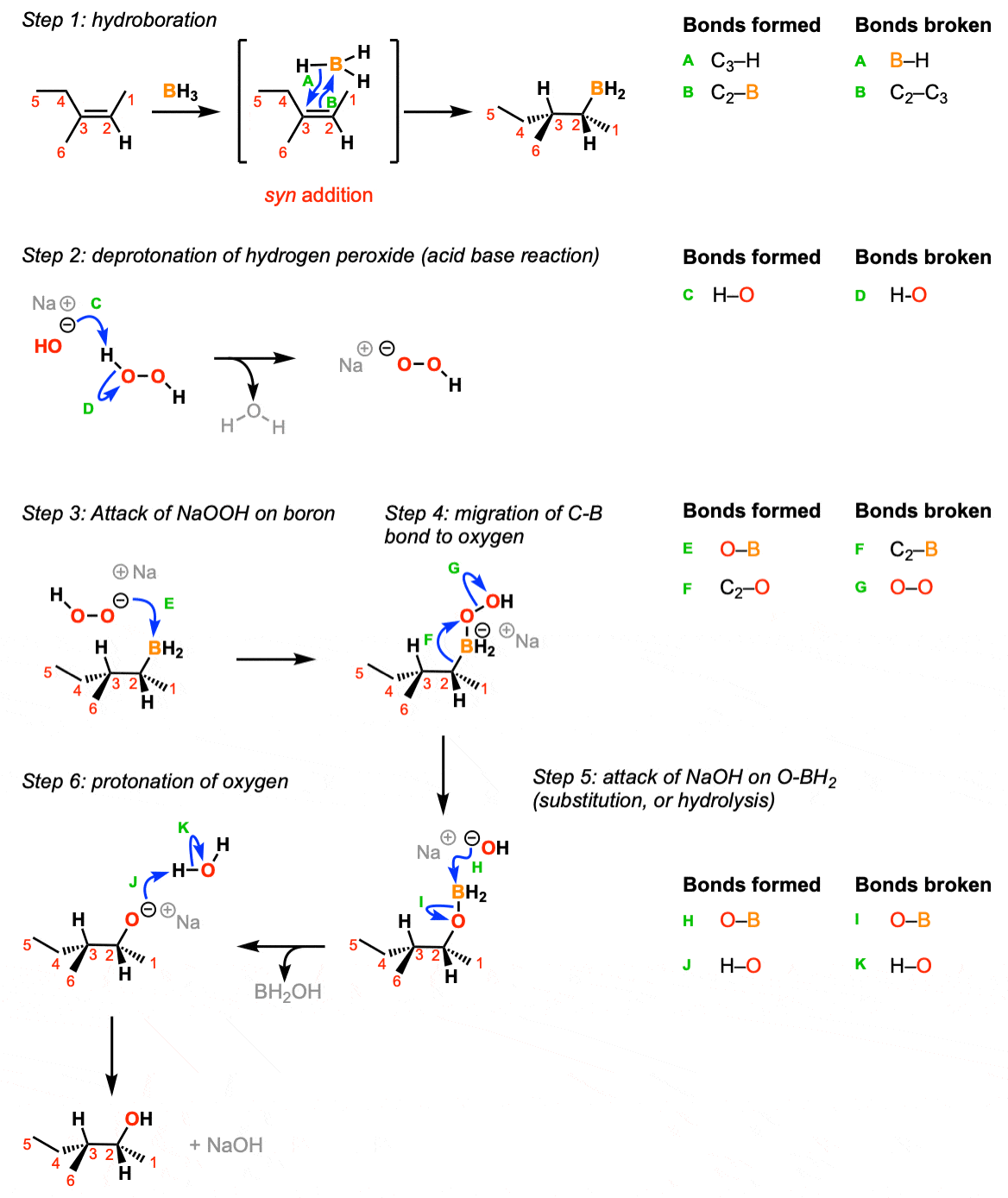
Notes: In step 1 the addition is syn and the reaction is concerted.
If excess alkene is present the two remaining B-H bonds can do subsequent hydroborations.
Note that only one enantiomer is shown here (but the product will be racemic)
The migration step (Step 4, arrows F and G) occurs with retention of stereochemistry at the carbon.
For the last step it’s reasonable to use water, HOOH or any other comparably acidic species as the acid.
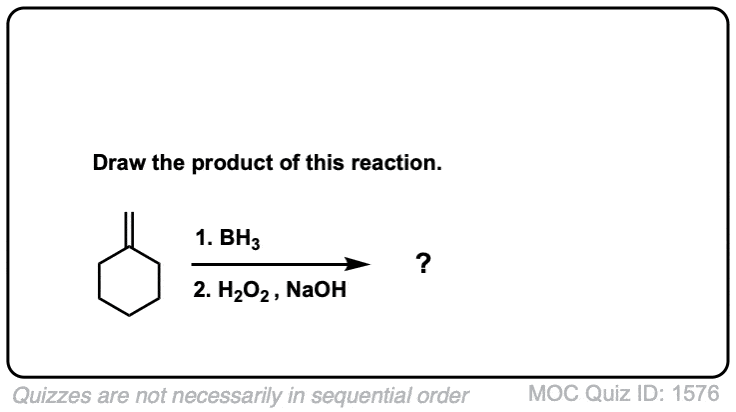
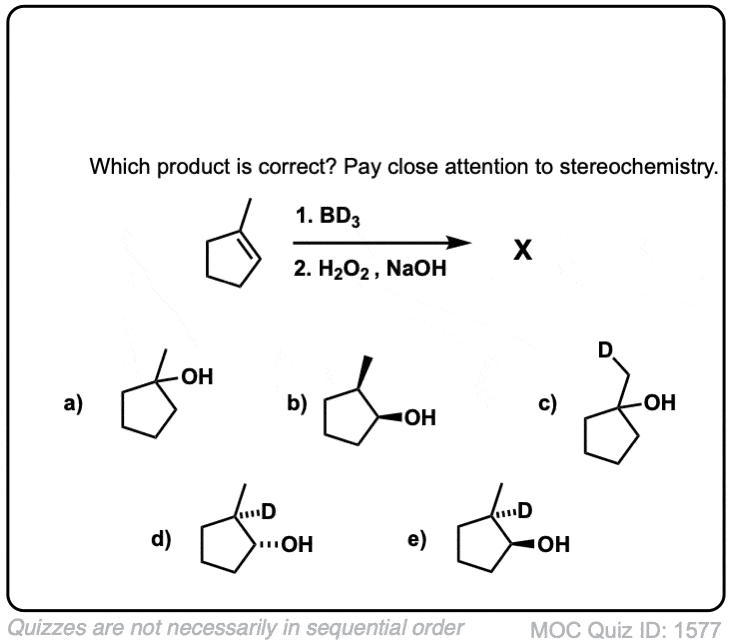
Quiz Yourself!
 Click to Flip
Click to Flip
 Click to Flip
Click to Flip
 Click to Flip
Click to Flip
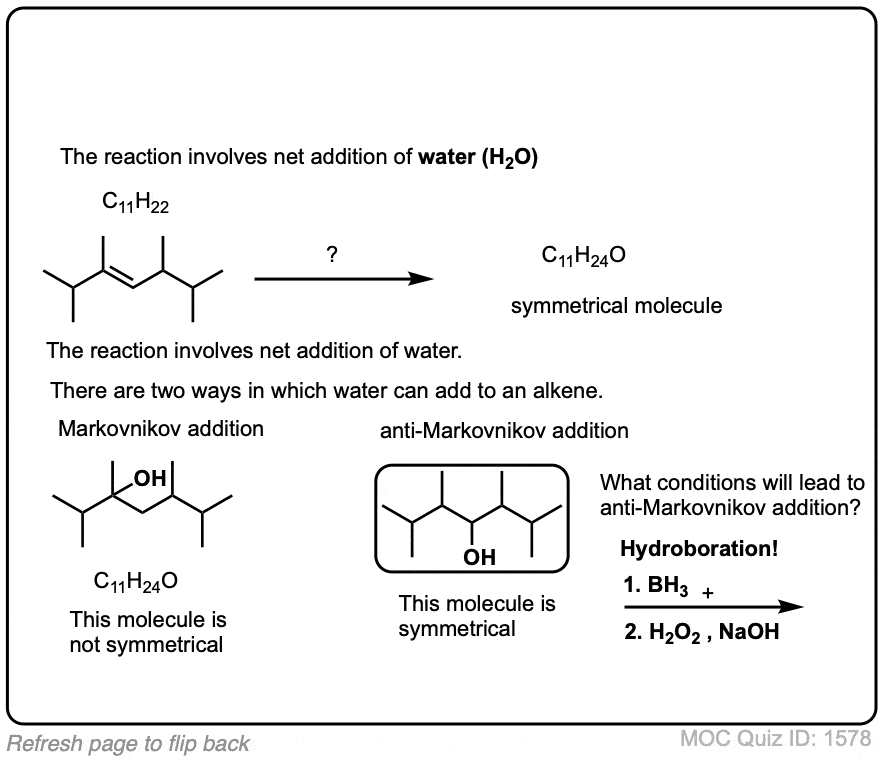
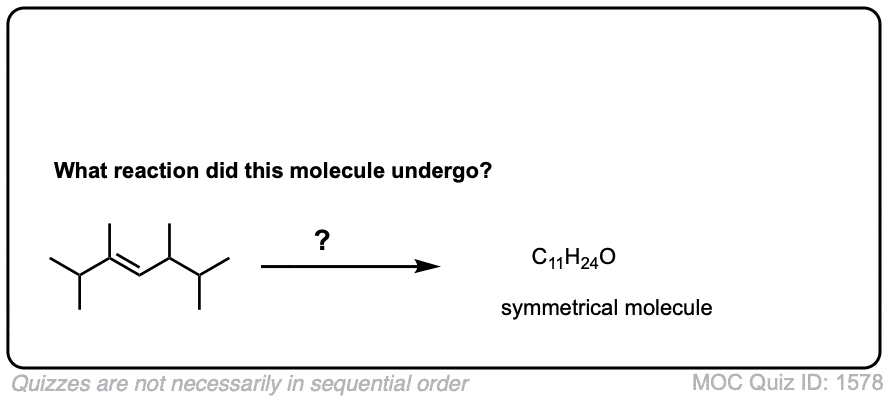
Exam-Type Examples
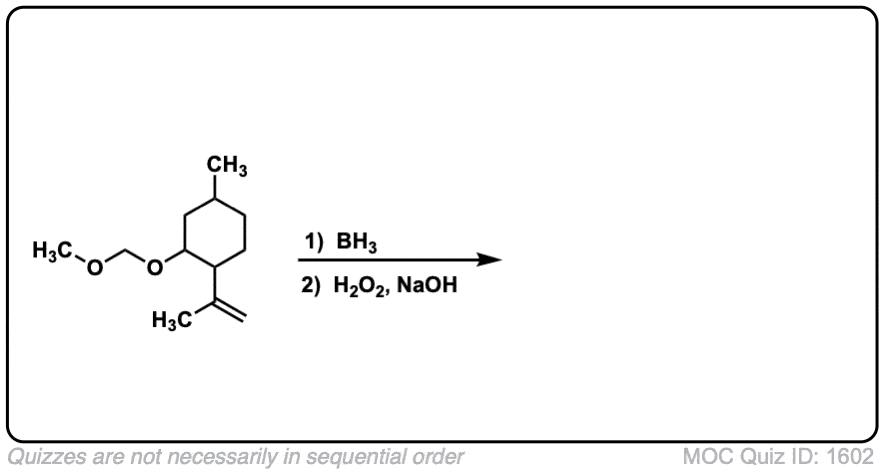 Click to Flip
Click to Flip
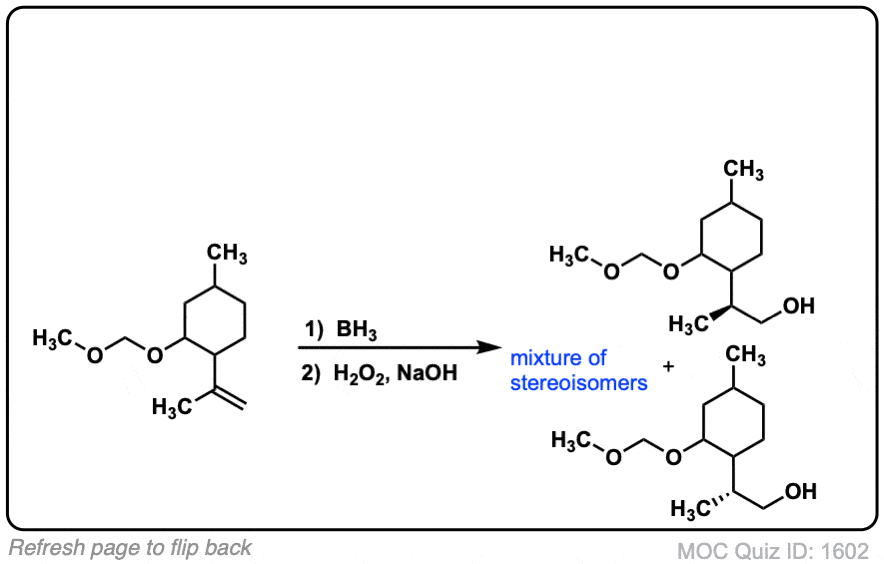
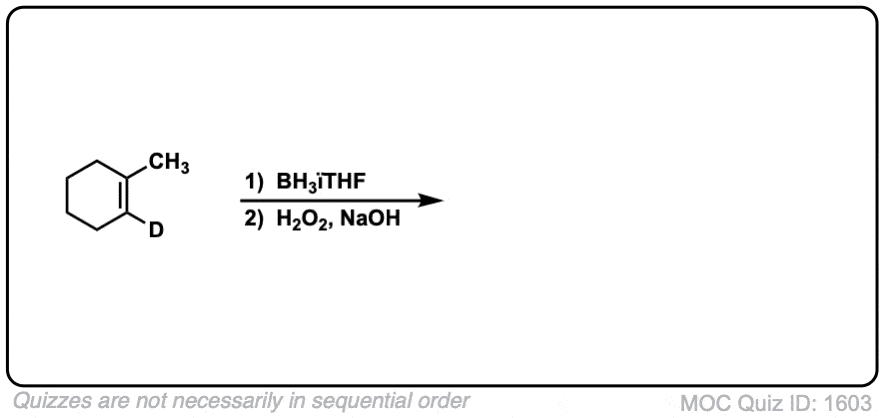 Click to Flip
Click to Flip
 Click to Flip
Click to Flip
 Click to Flip
Click to Flip
 Click to Flip
Click to Flip
 Click to Flip
Click to Flip
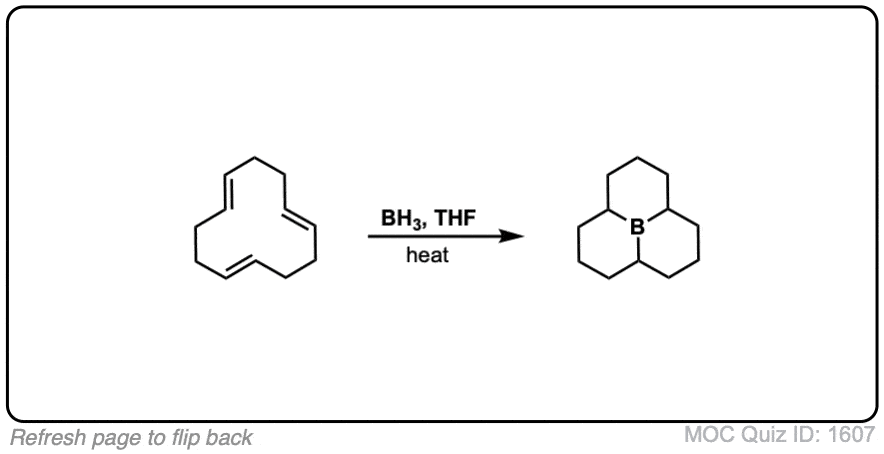
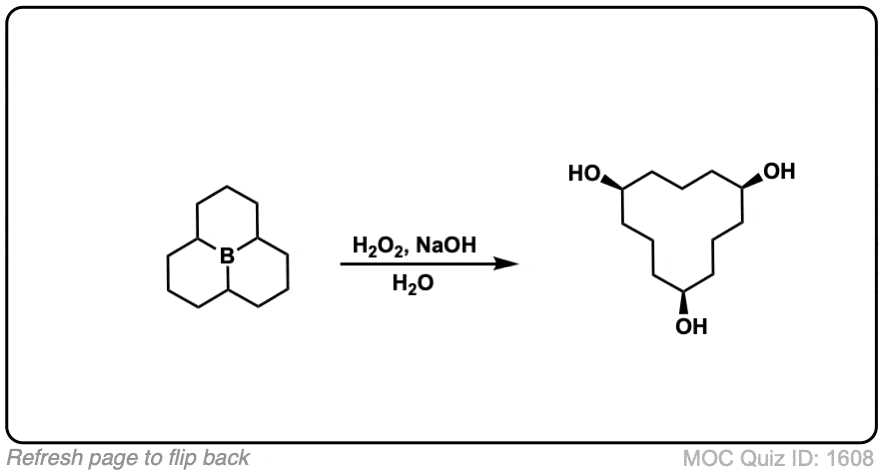
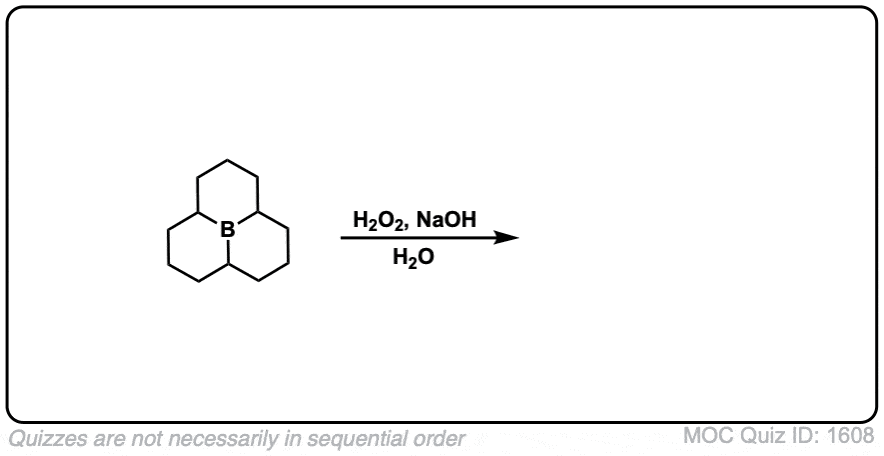 Click to Flip
Click to Flip
(Advanced) References and Further Reading
- First example
CONVENIENT NEW PROCEDURES FOR THE HYDROBORATION OF OLEFINS
Herbert C. Brown and George Zweifel
Journal of the American Chemical Society 1959 81 (15), 4106-4107
DOI:1021/ja01524a071
Early paper by Nobel Laureate H. C. Brown, describing variants of the classic hydroboration reaction. - Mechanistic studies
XXIV. Directive Effects in the Hydroboration of Some Substituted Styrenes
Herbert C. Brown and Richard L. Sharp
Journal of the American Chemical Society 1966 88 (24), 5851-5854
DOI: 10.1021/ja00976a029
A very nice Physical Organic study of the hydroboration of styrenes, involving a Hammett plot (a classic tool in physical organic chemistry) to determine a relationship between the stereochemistry of the reaction and the electron density of the alkene. - References from Organic Syntheses
A SIMPLE AND CONVENIENT METHOD FOR THE OXIDATION OF ORGANOBORANES USING SODIUM PERBORATE: (+)-ISOPINOCAMPHEOL
George W. Kabalka, John T. Maddox, Timothy Shoup, and Karla R. Bowers
Organic Syntheses, Coll. Vol. 9, p.522 (1998); Vol. 73, p.116 (1996)
DOI : 15227/orgsyn.073.0116
A Hydroboration-oxidation procedure where the oxidizing agent is sodium perborate, a cheap, safe, easily handled oxidant commonly used in laundry detergents. - Modern Example
Hydroboration with Pyridine Borane at Room Temperature
Julia M. Clay and and Edwin Vedejs
Journal of the American Chemical Society 2005 127 (16), 5766-5767
DOI: 1021/ja043743j
A modern method for doing hydroboration at room temperature using pyridine-borane, which usually requires heating to 75-100 °C to liberate the borane.
Shouldn’t there be 4 products for 4-methyl hex-2 ene?
No. The bulky hydroboration reagent 9-BBN is selective for hydroboration of the alkene in a manner that minimizes steric hindrance. In this case it orients itself such that the bulky boron reagent points away from the methyl group on the 4 position.
Hi i am jee aspirant and can u tell me how instead of adding naoh and h2o2, we supply heat and thus that compound form?? Like what is mechanism
Without having your specific example in front of me it’s hard to say what will happen, but in the absence of H2O2 or some other oxidant, no oxidation of the borane to the OH will occur.
Hie .Does the same mechanism happen when BH3:THF and H2O2 are reacted with the alkene in the absence of a base?If the same mechanism happens,where does the OH that causes the departure of alkoxide anion come from?
If base is not added, then the product is a boronic ester. Sometimes this is desired – for example, the boronic esters of pinacol and catechol are useful in many circumstances.
This article provides information about the hydroboration reaction using BH3 and its variants. It highlights that hydroboration follows an anti-Markovnikov addition pattern, and the stereochemistry is always syn. The mechanism of the reaction is also explained, involving several steps.
Why is hydroxide (OH-) needed for this mechanism?
Thank you!
One role for hydroxide is that it cleaves the B-O bonds and liberates the alcohol.
First, I LOVE your site. I refer my students to it regularly.
“One equivalent of BH3 can hydroborate up to 3 equivalents of alkene”
I think this is an odd statement. I think you mean, “One *mole* of BH3 can hydroborate up to 3 *moles* of alkene.” If I were to do a hydroboration, Equivalents are different. I would always want just over 1 equiv. of borane in a hydroboration. (Anything over 1 equiv. of a reagent in an organic reaction is excess and generally wasteful.) So, then I would do the calculation based on the fact that 1 equiv. of borane will be 1/3 the number of moles of alkene used in the reaction. Right?
Maybe a minor point.
On my book, it says that 3 alkenes react with BH3, then oxidation with peroxide, finally hydrolysis with water to get 3 alcohols… Now I don’t know which one is correct, or are they both correct
Yes – BH3 can react with up to 3 alkenes. It depends on how many equivalents of BH3 are used relative to the alkene. I’m not sure how it’s tested in your course but for practical purposes it’s generally fine to show BH3 reacting with one alkene or three alkenes.
Does the reaction change if THF is present with the BH3? Or is that a different reaction all together?
Same reaction. No difference.
In the first example, does the C where OH adds has to be a stereocenter for there to have a stereoselectivity? Thank you in advance!
A different way of asking the same question, which is the question I think you’re getting at, is, “how do you know if this will reaction will give you an achiral product, a racemic mixture of enantiomers, or a mixture of diastereomers.”
My answer to this is to apply the pattern of this reaction to the alkene. 1) the bond forming/breaking pattern; break C-C, form C-H and C-OH, putting C-OH on the least substituted carbon of the alkene. 2) apply the stereochemistry pattern, putting C-H and C-OH on the same face of the alkene. 3) keep in mind the reagent can add to both faces of the alkene. So draw out the product that would have arisen from attacking the opposite face.
Now compare these two molecules, as you would when trying to answer, “enantiomers, diastereomers, or the same” ? https://www.masterorganicchemistry.com/2019/03/08/enantiomers-diastereomers-or-the-same-1-using-models/
Can we get territory alcohols from hydroboration oxidation of alkenes
Tertiary? Yes, of course, although it would be of a tetrasubstituted alkene.
How we get t-alcohol by hydroboration-oxidation of alkene!!!
Well the obvious precursor is 2-methyl-prop-2-ene. But hydroboration won’t get you there; use hydration or oxymercuration instead.
What if instead of BH3 you have B(CH3)2H?
Dimethylborane? It will perform one hydroboration and stop there. With BH3 it could perform up to three hydroborations.
Hey what happens when BH3 reacts with alkynes? In presence of THF and CH3COOH?
Hydroboration of alkynes usually generates aldehydes/ketones depending on the structure of the alkyne. After hydroboration-oxidation, Terminal alkynes give aldehydes, internal alkynes give ketones. However the best reagent for that is not BH3 but a disubstituted borane like 9-BBN, dicyclohexylborane, or disiamyl borane.
Are you sure it’s CH3COOH and not CH3COOOH (a peroxyacid) which would do the oxidation step?
Thank you so much for this! Even helped me in pharmacy school!!
OK! Great to hear Andrea!
How does step 5 lead to step 6.. ? I mean at first carbon is directly bonded to boron and then changes direct bond to oxygen… How does the bonds invert ??
It’s a rearrangement step. Very similar to the 1,2- shifts you see on carbocations such as hydride and alkyl shifts. The pair of electrons in the sigma bond is acting as a nucleophile.
Hi! I just got my exam back and for some reason my professor took off points because I used 1)BH3*THF 2)H2O2, OH instead of 1)9-BBN 2) H2O2, OH. Our starting material was hex-1-yne and product was pentanal… If both of these reagents do the exact same thing…
* I am not quite sure I understand where I went wrong or why I lost points. Thanks!
Yes. I need to fix this. In the hydroboration of alkynes with BH3, hydroboration can happen multiple times. Something like 9-BBN or diisoamylborane is preferable for this reason.
Hi After the reaction of alkene with BH3, please can you show a step in a case where you are asked to hydrogenate the product before oxidation with hydrogen peroxide
Hydrogenation of the product of alkene hydroboration? I don’t follow, because there won’t be any double bonds left. Perhaps you’re referring to the reaction with alkynes?
Hi James!
i was told that in HBO (Hydroboration-Oxidation) reaction, the H added comes from BH3 whereas OH comes from H2O2. However, after looking at your mechanism, it seems like the ‘H’ in OH (last step of mechanism) comes from H2O rather than H2O2. This has put me in a kind of fix as to what is right.
(Wikipedia specifically mentions this – The ‘H’ atom in the reaction comes from B2H6 while the ‘OH’ atom comes from hydrogen peroxide (H2O2))
So what’s going on here? kindly help!
Rupinder
The H that forms the C-H bond comes from boron (BH3). No question about this.
The O in the C-O bond comes from hydrogen peroxide. The H on the alcohol (OH) likely comes from the solvent (H2O) that serves to protonate the oxygen during the process of hydrolyzing the boron off, although in solution, the H on OH groups can move around a bit in a process known as “exchange”.
Why is that -O-O-H acts as the nucleophile in the oxidation step. Isn’t OH- a better nucleophile due to its smaller size and greater electron density on attacking O (less -Inductive effect of other O as in OOH) ?
-OH is perfectly capable of acting as a nucleophile on B, but the problem is that nothing happens. It’s an equilibrium reaction, and the -OH will eventually depart. It’s only when -OOH adds to B that the rearrangement step can occur.
In general peroxide is a better nucleophile than OH due to a phenomenon called the “alpha effect’, but this isn’t generally covered in introductory courses. Organic chem is full of counter-intuitive surprises!
I was under the impression that for Step 5 there are a series of oxidizing reactions on organoborane until it finally leaves via E1 to form a more stable alkoxide in a basic solution. Am I correct on this or does this not happen in this reaction? Thanks.
There are definitely a series of oxidizing reactions on the organoborane, until it becomes a boronic ester (i.e. all B-O bonds, no B-R bonds). I’m not sure I follow re: “the E1 step”. If you mean formation of a tetrahedral, charged borate intermediate followed by loss of the alkoxide as a leaving group (somewhat similar to the first step in the SN1 or E1 reactions) then yes!
In step 5, what happens to the BH2 group after it is attacked by OH-?
In this sequence, it becomes H2B-OH . However I should note that all the B-H bonds are available for hydroboration of other alkenes; in the end, what will happen is that we form B(OH)3 (boric acid).
thx james !
B(OH)3 WILL be formed when we have for example 3 cyclohexens right ?
but my question is :
in some books they put THF as a solvent . and they say that we will get a BH3-THF complex from that because bore is very reactive as lewis acid so what we will get from that ? could not we use instead of THF the DMSO according to you ?
please reply
BH3-THF should not react significantly differently from BH3-DMS complex.
Yes, B(OH)3 will be the eventual fate of boron from hydroboration-oxidation.
Hi, I’m wondering, how come O and BRH2 get swapped between step 4 and 5?
There doesn’t really seem to be an explanation that I can see for that…
Hi Peter, in this key rearrangement step, we’re breaking the C-B bond and forming a C-O bond, which happens also to break the weak O-O bond on the peroxide.
Just kind of confused as to the greyed out Na+, is it just kind of floating around getting transferred between the molecules, or does it only really come in at step 5 and 6?
Thank you :)
HR
Is there a reason borane specifically adds to the least substituted alkene side..
I was trying to reason it out. It is a electrophilic addition because Boron is electron deficient(?). But that doesn’t explain why the end-product is more stable.
I could ask my prof this but already he thinks I have too many q’s outside the scope of this class.
Usually the less substituted carbon gets the negative charge on electromerisation. So the the empty orbital of boron comes and gets attached to the “less substituted carbon”. The moment this happens, the H-B bond breaks and the H- ion gets attached to the neighbouring C which had positive charge due to electromerisation. And all this happens in a single concerted step. The product you get is the third compound mentioned in the step 1 illustration.
this post is like a medicine for students who are sick with the difficult bookish language. thanx james
This complete mechanism is shown in few textbooks, yet it is one of the important anti-Markovnikov syn-addition. Most authors don’t bother to explain the oxidation part that yields the final OH group.
Hi James,
Is carbocation rearrangement possible? In adding H-Cl, there is a carbocation, but it appears that there isn’t one here.
So I see how this can result in racemic mixtures for an alkene, and I’m wondering – Is there a way to then filter out one specific enantiomer, so I can get only the R or S enantiomer?
Thanks!
J
Thank you SO much for this. My organic book and the other four organic texts I got from the library explain this in the most cryptic way ever. I’ve spent HOURS trying to find a coherent explanation for this mechanism. You are a LIFE saver!
Please, never take this site away! At least not until I get into med school anyways! :)
Thanks!
try morrison boyd
very informative and helpful for students like me. Thankyou!!
Could you explain the relationship between hydroboration and free radical addition (peroxide effect) please?
Well, they are both reactions in which the hydrogen adds to the less substituted end of the double bond (so-called “anti-Markovnikov addition”) but the similarity ends there as their mechanisms are very different.