SN2 Reaction of Acetylide Ions with Alkyl Halides
Description: Alkyl halides treated with acetylide ions (the conjugate bases of acetylenes) will undergo SN2 reactions to give alkynes.
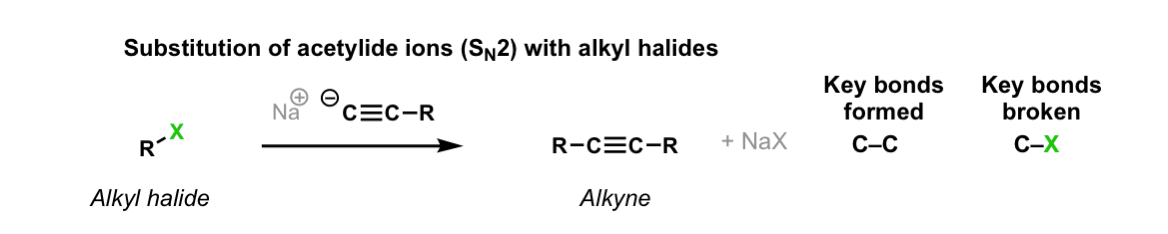
Notes: This works best for primary or methyl alkyl halides. Sodium (Na) is not crucial, it’s just a spectator ion.
See also: Deprotonation of alkynes with strong base to give acetylide ions.
Examples:
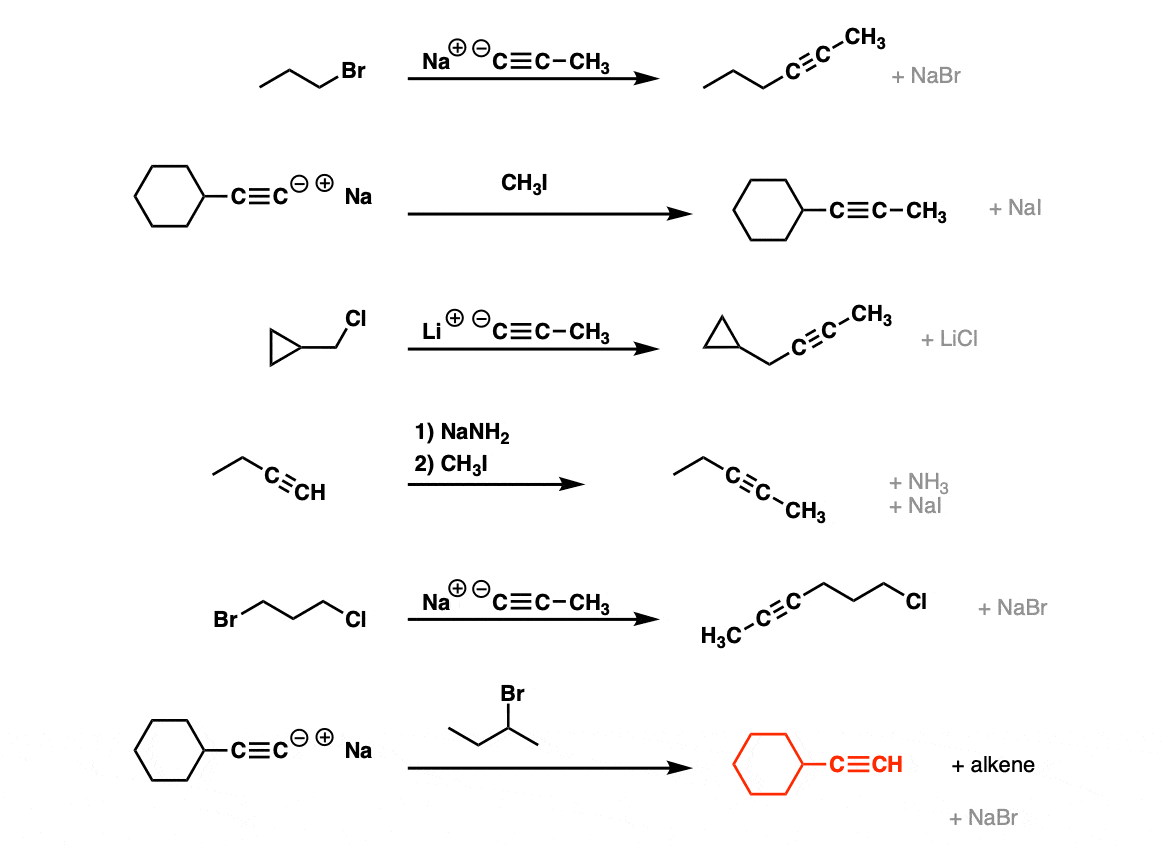
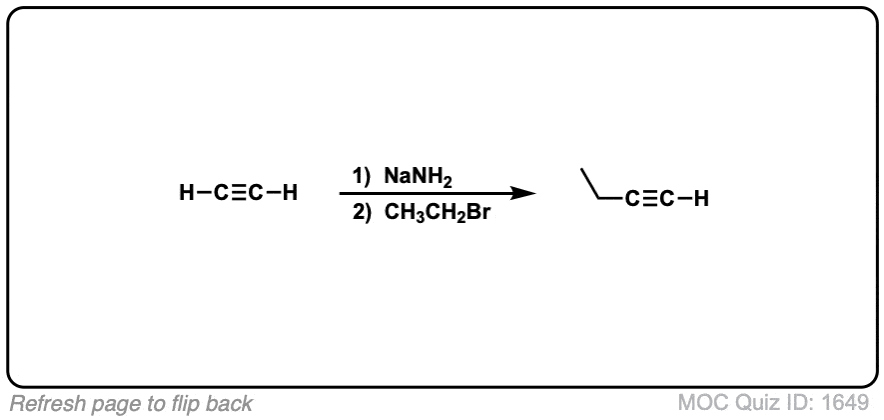
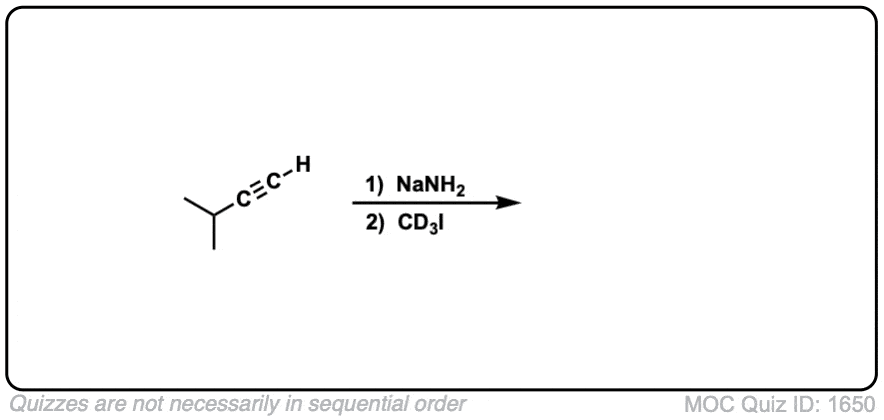
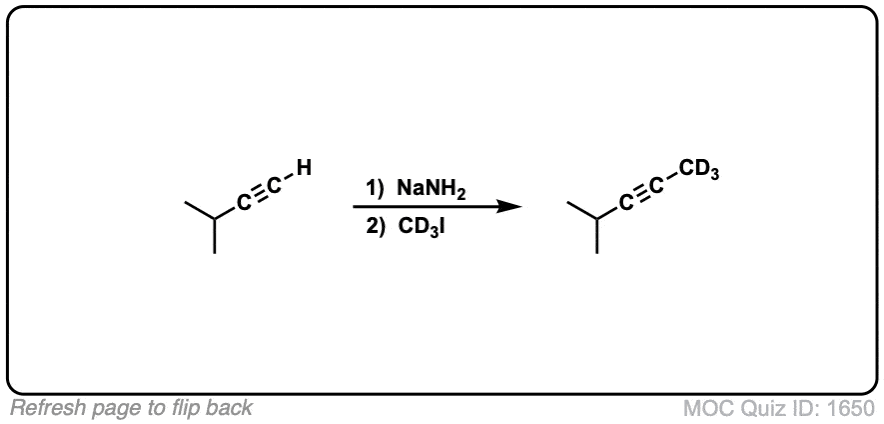
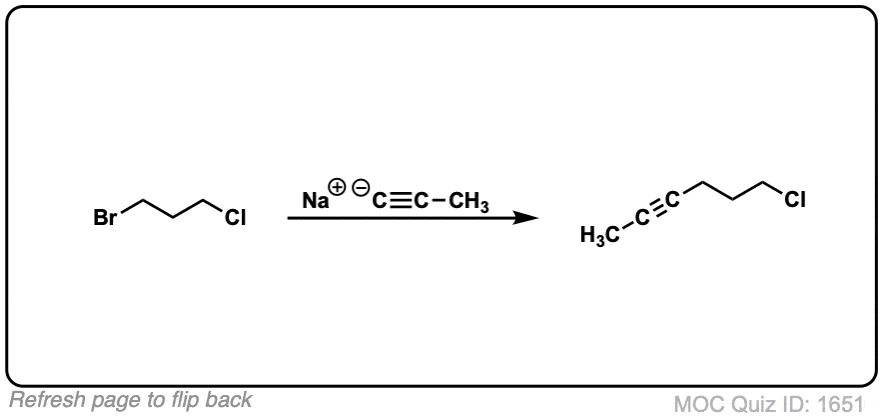
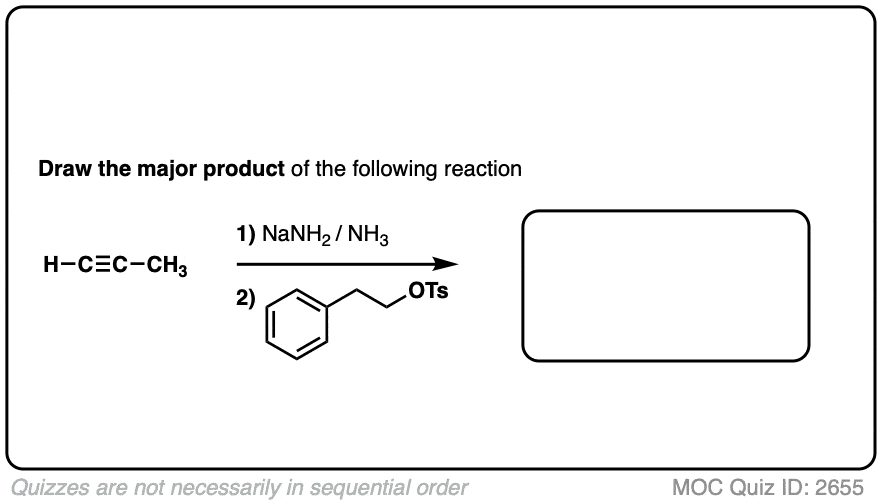
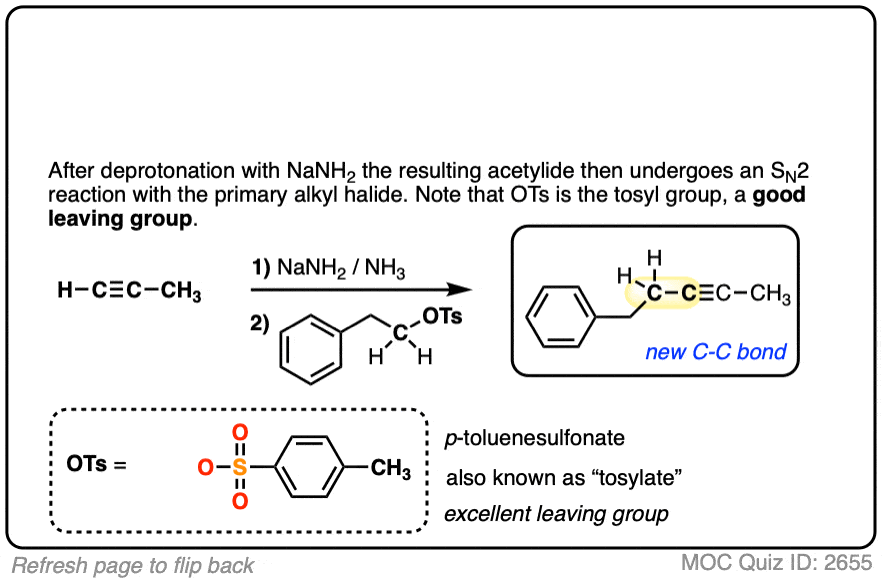
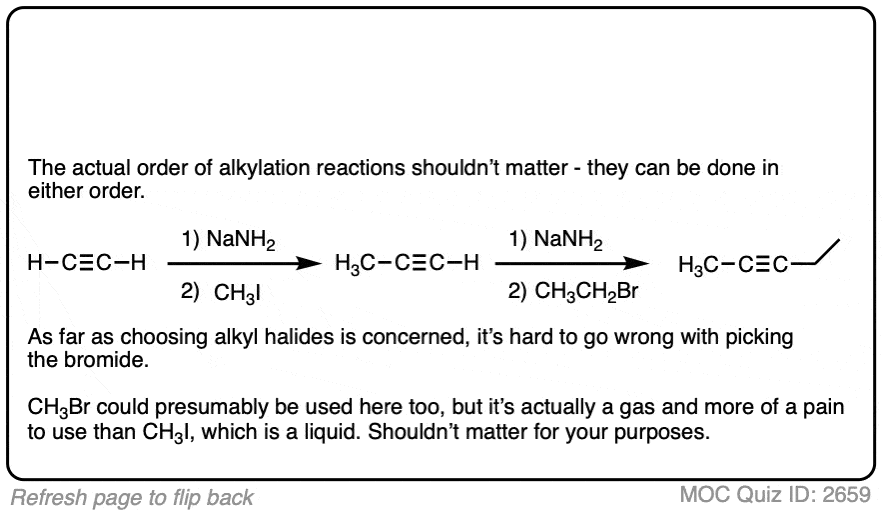
Notes: Be prepared to see the alkyl halide either as the reactant (example 1) or above the arrow (example 2). It is also common to see the alkyne as starting material with a two-step deprotonation/SN2 process (example 4).
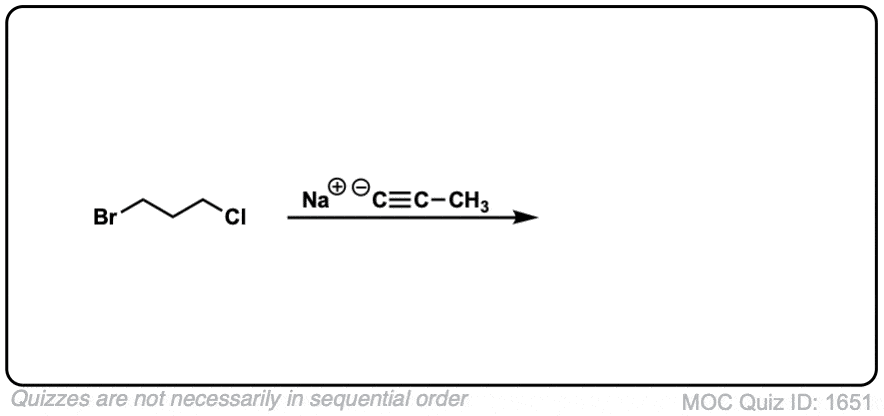
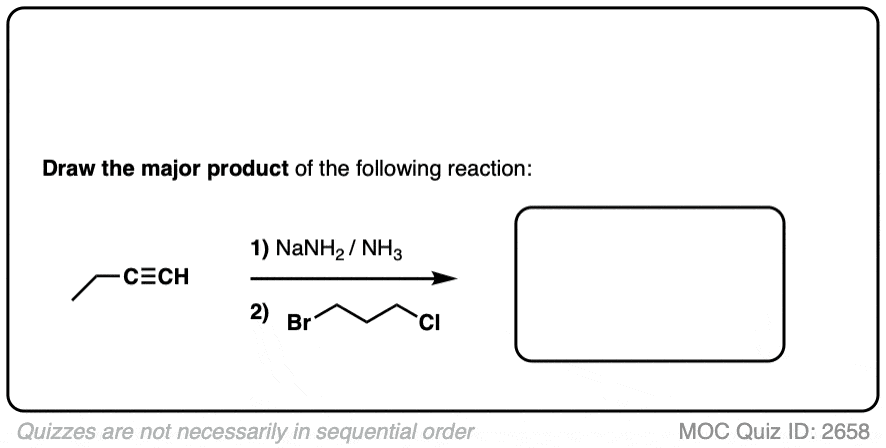
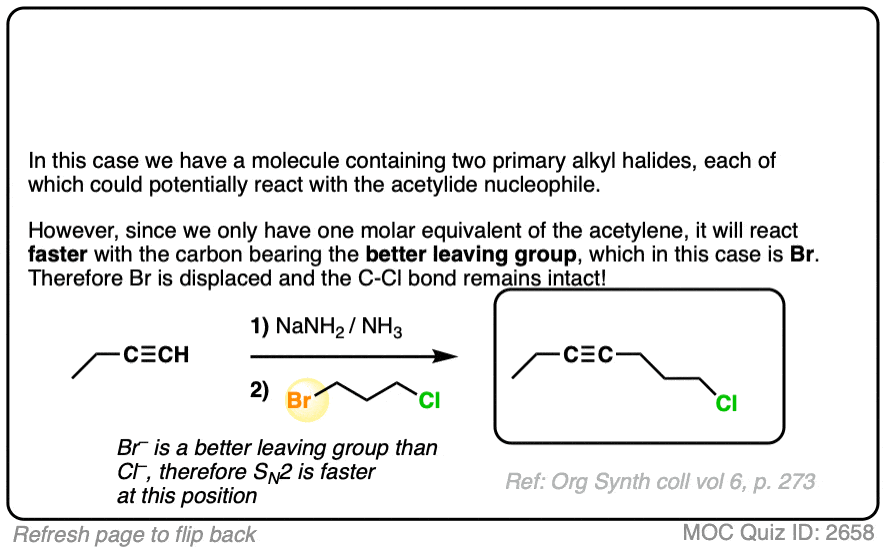
Since this is an SN2 reaction, everything you’ve previously learned about SN2 reactions still applies. Example 5 with one equivalent of nucleophile will react preferentially to displace the better leaving group Br(-).
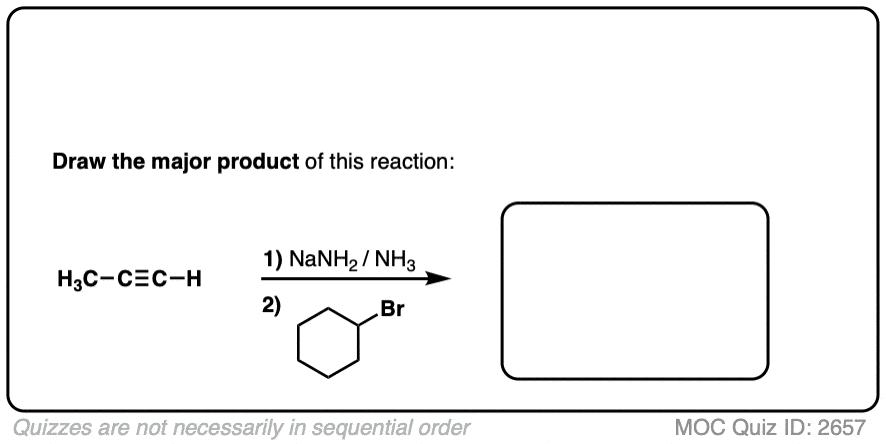
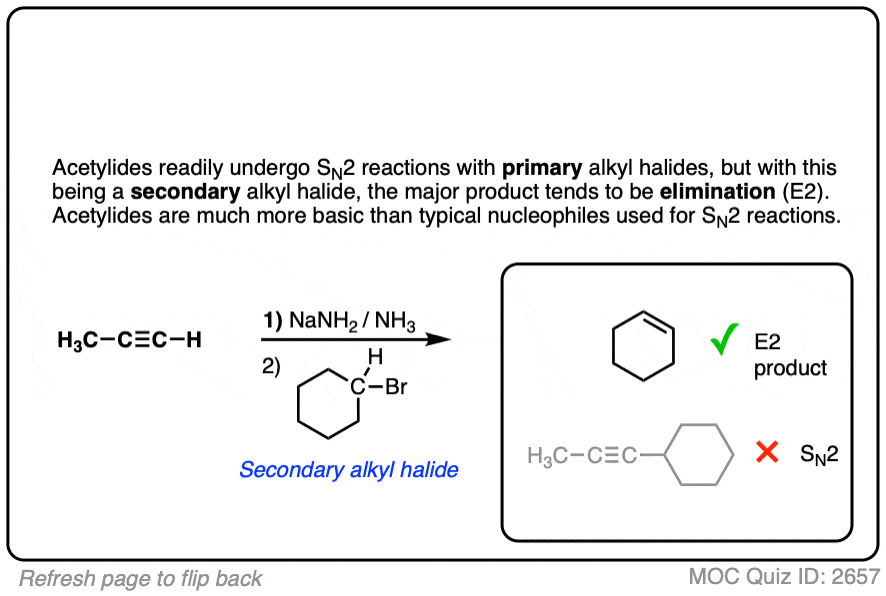
Finally this reaction works best for primary alkyl halides. Since the acetylide is such a strong base, secondary and tertiary alkyl halides tend to get deprotonated instead (example 6).
Mechanism: Acetylide ions are great nucleophiles. They will perform SN2 reactions on alkyl halides (Step 1, arrows A and B).
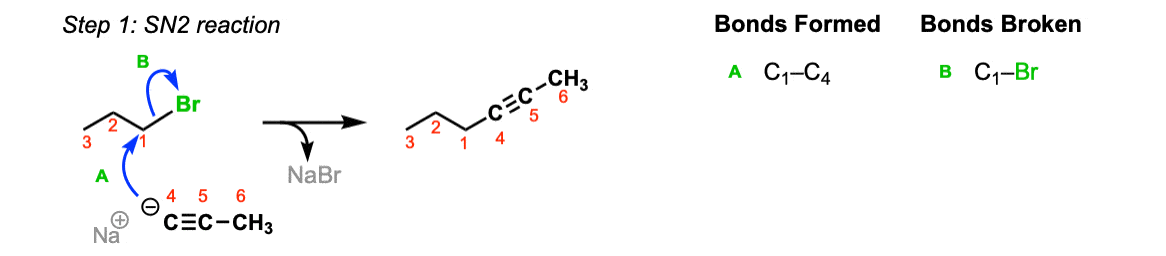
Notes: Really important! There aren’t many ways of making C–C bonds in Org 1, but this is one of them. This is one of the most USEFUL ways of making C–C bonds. Furthermore, alkynes are really versatile and can be turned into all kinds of other functional groups.
See: Alkynes Are A Blank Canvas
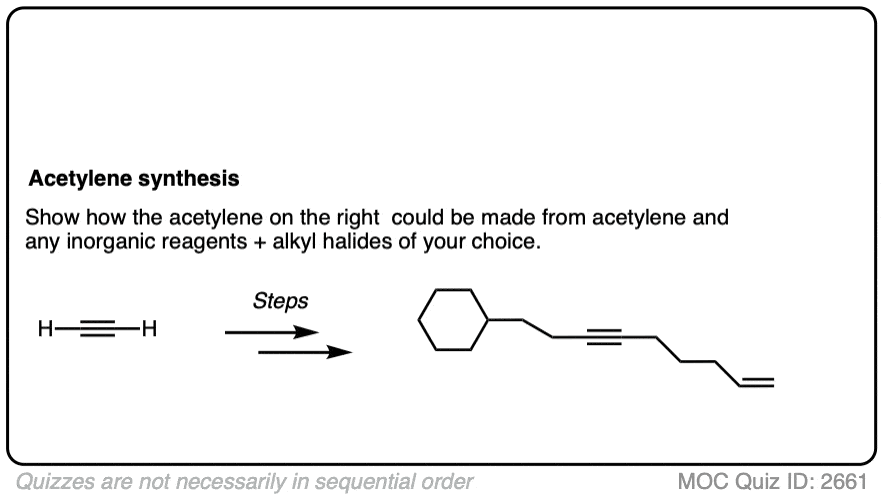
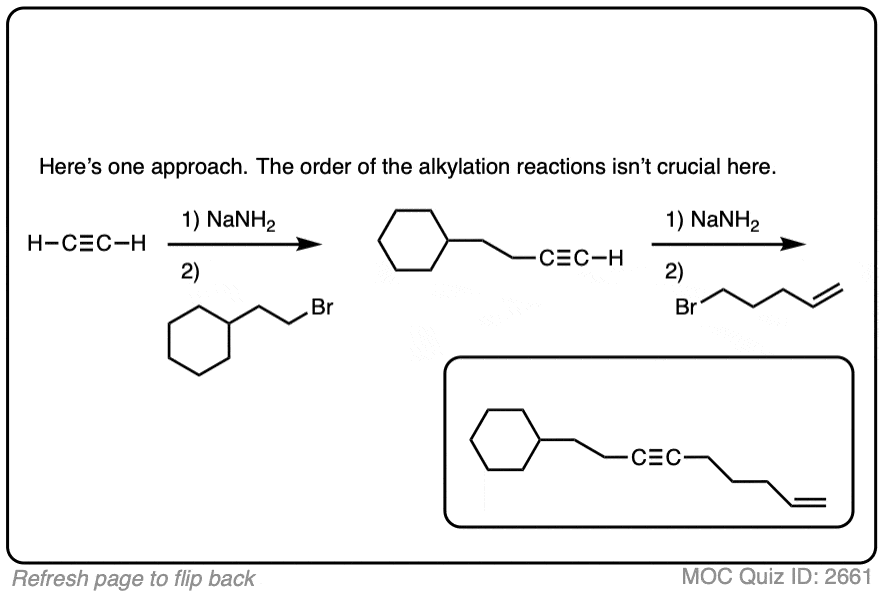
Quiz Yourself:
 Click to Flip
Click to Flip
 Click to Flip
Click to Flip
 Click to Flip
Click to Flip
 Click to Flip
Click to Flip
 Click to Flip
Click to Flip
 Click to Flip
Click to Flip
 Click to Flip
Click to Flip
 Click to Flip
Click to Flip
 Click to Flip
Click to Flip
 Click to Flip
Click to Flip
(Advanced) References And Further Reading
This is a classic reaction taught to undergraduate students taking organic chemistry. Its utility lies in the fact that it allows a simple way to form C-C bonds.
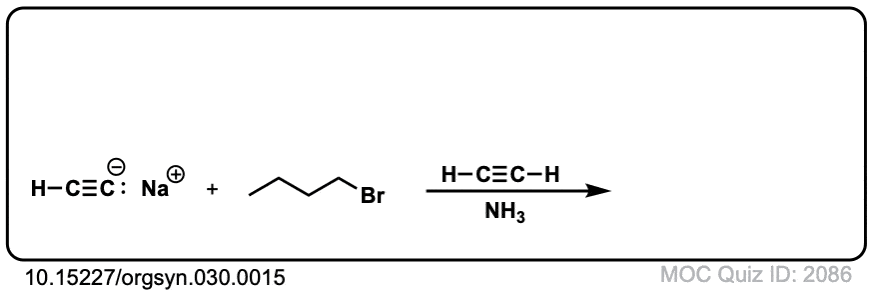
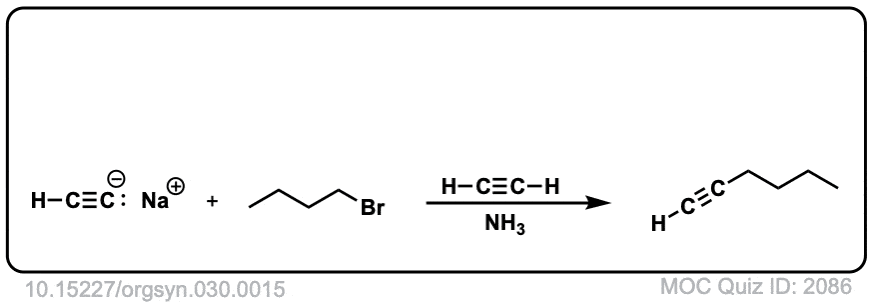
- n-BUTYLACETYLENE
Kenneth N. Campbell and Barbara K. Campbell
Org. Synth. 1950 30, 15
DOI: 10.15227/orgsyn.030.0015
An extremely simple example of this reaction. The deprotonation is done with Na metal in liquid ammonia, and care has to be taken to avoid the conditions of dissolving metal reduction (the procedure states that the reaction should not turn blue). - Synthesis of Unsymmetrical Alkynes via the Alkylation of Sodium Acetylides. An Introduction to Synthetic Design for Organic Chemistry Students
Jennifer N. Shepherd and Jason R. Stenzel
Journal of Chemical Education 2006, 83 (3), 425
DOI: 10.1021/ed083p425
A nice paper that describes the adaptation of this reaction for undergraduate teaching labs.
Real-Life Example:
Org. Synth. 1950, 30, 15
DOI Link: 10.15227/orgsyn.030.0015
 Click to Flip
Click to Flip
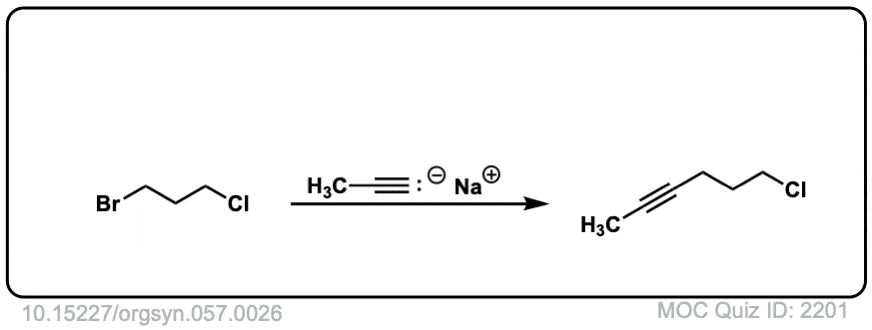
Org. Synth. 1977, 57, 26
DOI Link: 10.15227/orgsyn.057.0026
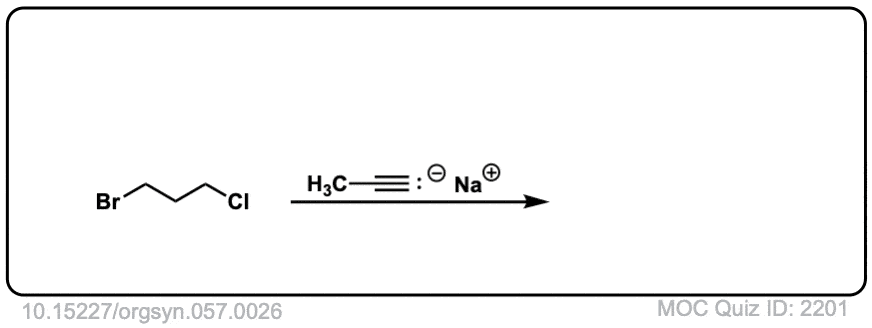 Click to Flip
Click to Flip
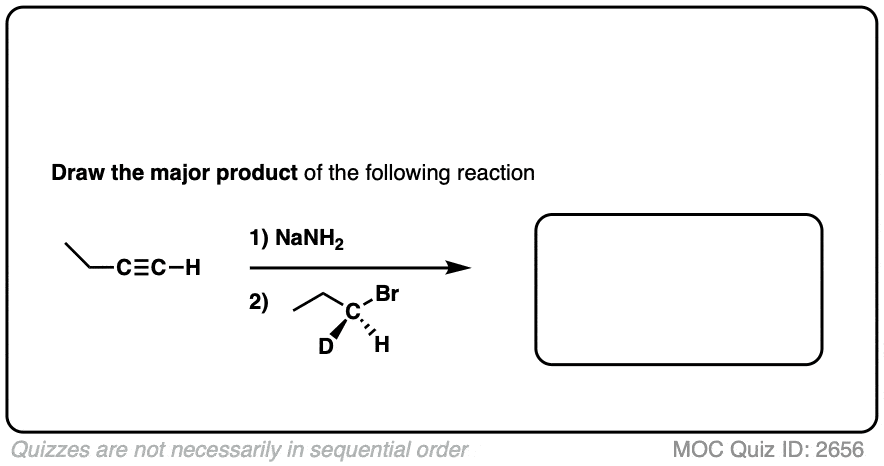
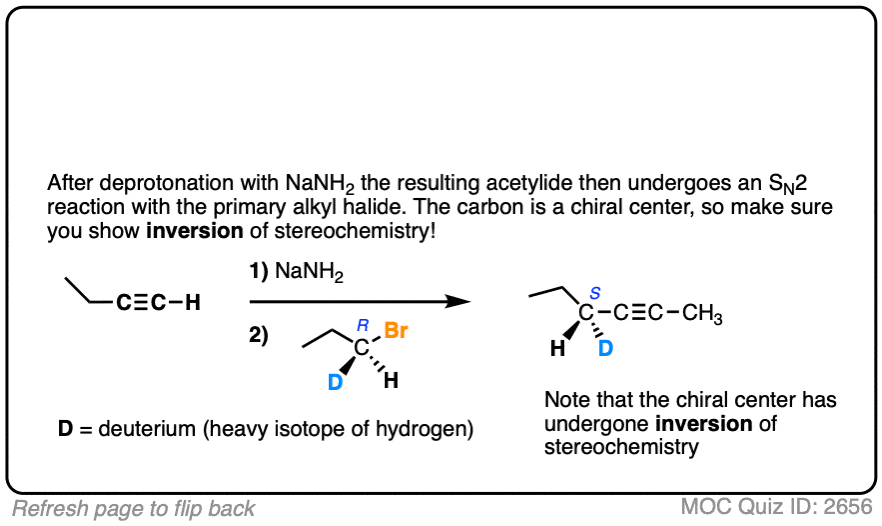
Hi Mr. James, I think that you had a tiny typo at Quiz ID 2656: the reactant at step 2 should be (S)-1-bromopropane-1-d, instead of (R)-1-bromopropane-1-d.
Fixed. Thank you so much!
Hello,
I was wondering how does acetylene react in front of an hindered R1.
Is the reaction an SN2 or an E2 ?
Thanks
With primary alkyl halides – SN2. With secondary alkyl halides – E2
Example 6 (as pointed out before) has a typo, it says NBr instead of NaBr
Fixed. Thanks for letting me know!
Hi!
In example 6, from where do the hydrogens that get added to the acetylide ion and alkane come from?
With secondary alkyl halides, acetylides tend to act as *bases* rather than perform SN2 reactions. So this is actually an E2 reaction!
Shouldn’t example 6 be +NaBr instead of +NBr?
With secondary alkyl halides, acetylides tend to do E2 reactions, not SN2 reactions!
Yes, fixed. Thanks!
From where exactly are you getting the arrows C,D,E,F,G,H in this mechanism
Fixed. thanks.