SN2 reaction of amines with alkyl chlorides to give ammonium salts
Description: Amines will react with alkyl halides to give ammonium salts. Importantly, this occurs even if only one equivalent of the alkyl halide and amine are used.
The rest of this page is available to MOC Members only.
To get access to this page, plus over 2500 quizzes, the Reaction Encyclopedia, Org 1 / Org 2 summary sheets, and flashcards, sign up here for only 30 cents/ day!
Real-Life Examples:
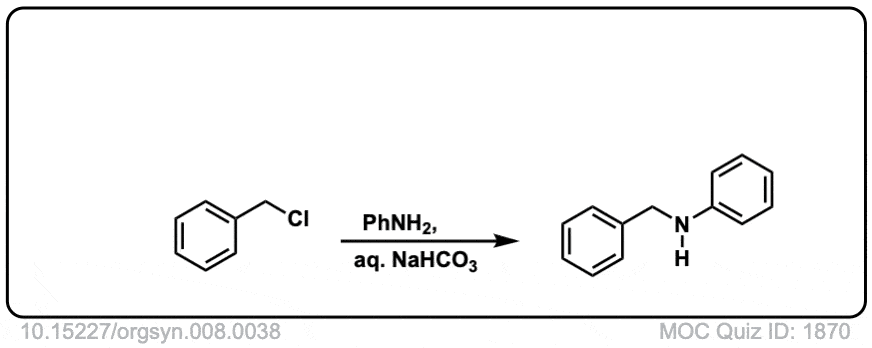
Org. Synth. 1928, 8, 38
DOI Link:10.15227/orgsyn.008.0038
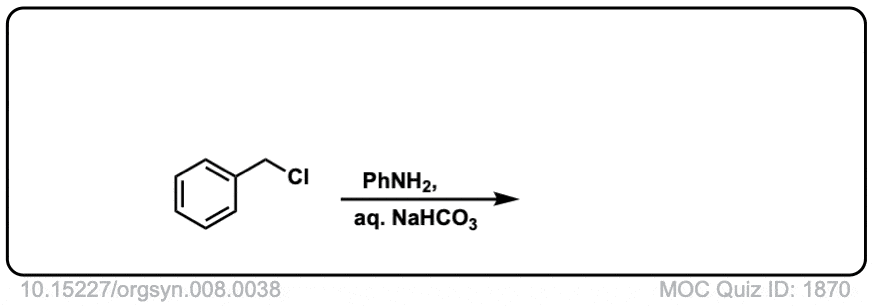 Click to Flip
Click to Flip
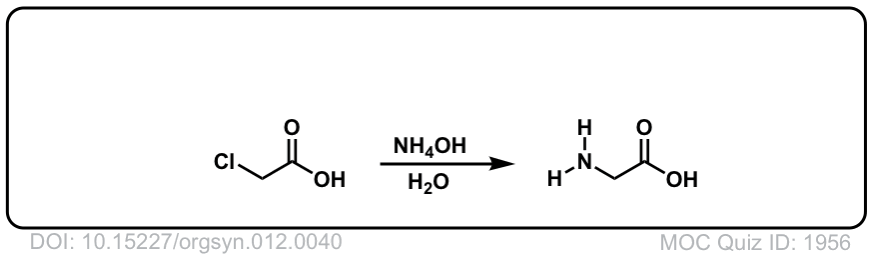
Org. Synth. 1932, 12, 40
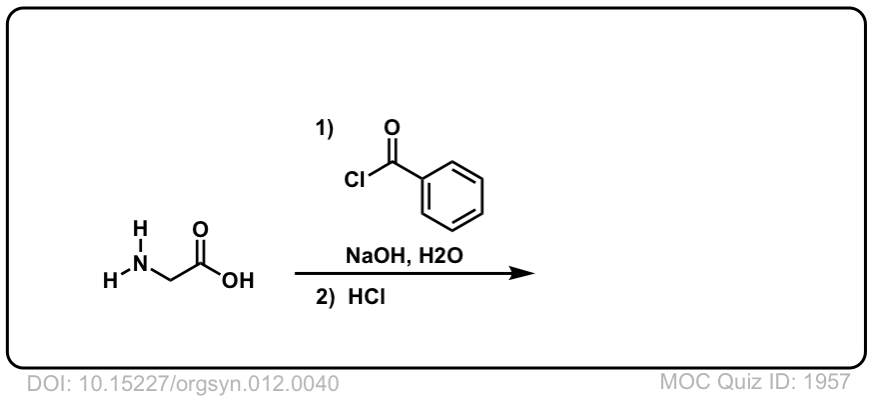
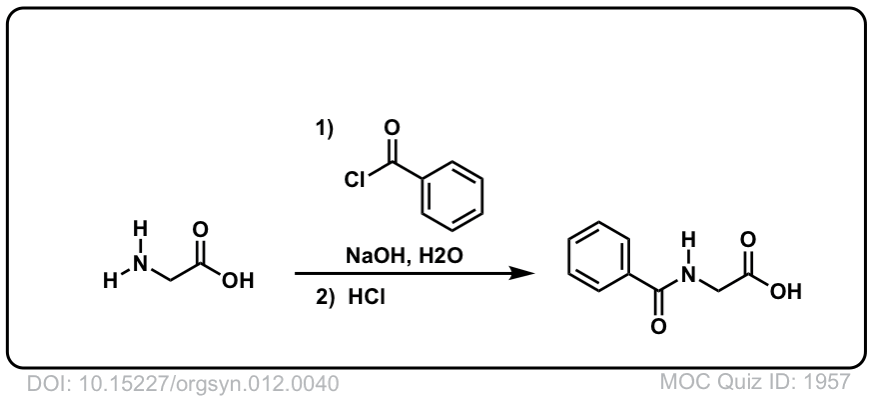
DOI Link:10.15227/orgsyn.012.0040
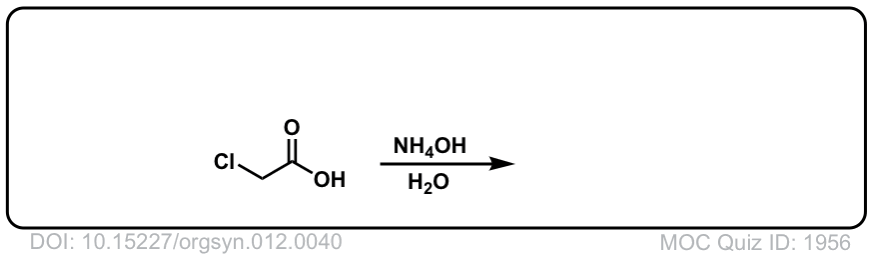 Click to Flip
Click to Flip
Org. Synth. 1932, 12, 40
DOI Link: 10.15227/orgsyn.012.0040
 Click to Flip
Click to Flip
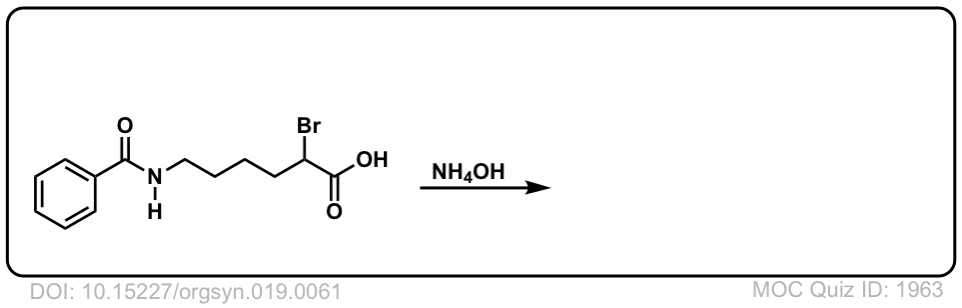
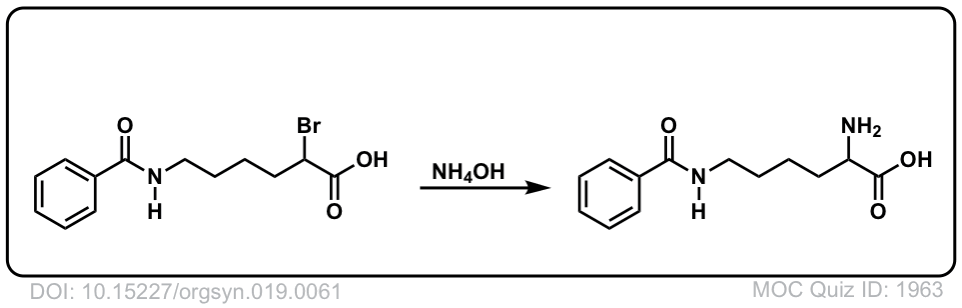
Org. Synth. 1939, 19, 61
DOI Link: 10.15227/orgsyn.019.0061
 Click to Flip
Click to Flip
Org. Synth. 1938, 18, 56
DOI Link: 10.15227/orgsyn.018.0056
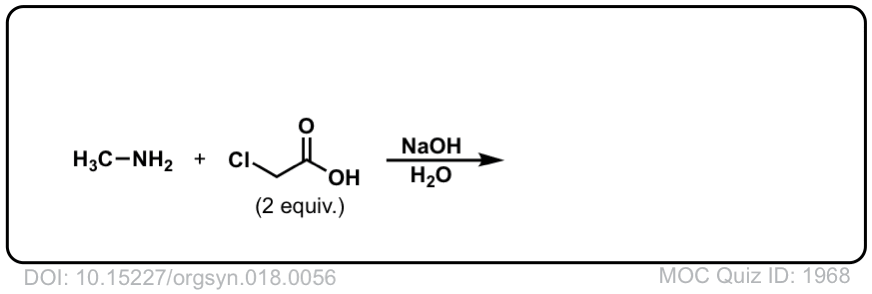 Click to Flip
Click to Flip
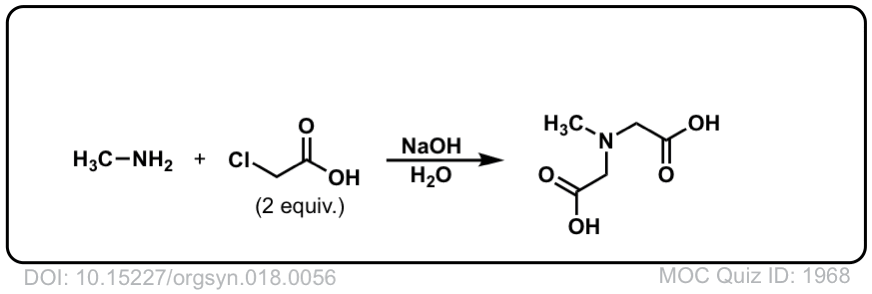
Hi,
is it possible to eliminate the quaternary ammonium ion?
Yes, that’s the Hofmann elimination. https://www.masterorganicchemistry.com/2017/10/18/the-hofmann-elimination/
Hi, in Description is written “+ salt”. What kind of salt is created? I suppose that there is 2 HX? Thx, P.
The “salt” is the ammonium salts that form during deprotonation of the amine.