Org 1 Summary Sheets
Org 1 Summary Sheets – A 20-Page PDF Summary of A First-Semester Organic Chemistry Course
- These sheets provide a condensed summary of an entire Org 1 course, so you can access the key concepts, reactions, and mechanisms from your textbook in just a few pages.
- If your midterm or final is fast approaching, and you feel behind in your exam prep these notes can help you get up to speed – fast!
- Full-color, printable PDF file. No physical item will be mailed to you.
- Designed for introductory organic chemistry courses at all levels in the USA and Canada (including ACS exams), but also useful as a refresher on the basics!
These sheets are included with the MOC membership. (Alternatively they can be purchased separately from the online store. )
MOC members will see a download link below (must be logged in to see it)
[Download link hidden - Join here]
What’s Included in the Org 1 Summary Sheets
Hybridization • Formal Charge • Nomenclature • Conformations • Cycloalkanes • Resonance • Free Radicals • Acids and Bases • Functional Groups • Stereochemistry • Substitution and Elimination • SN1/SN2/E1/E2 Decision • Addition to Alkenes • Reactions of Alkynes • Reaction Energy Diagrams • Synthesis • Alcohols and Ethers
- Introduction to Alkane Nomenclature (free sample download ) – How to name organic compounds – Functional group priorities – Applying the chain length rule – Identifying substituents – The lowest locant rule – Dealing with multiple and branched substituents – Putting it all together

- Bonding and Hybridization – What is bonding and why does it occur – What is antibonding – What is pi bonding – Hybridization – sp3, sp2, and sp hybridization examples – Trigonal pyramidal, trigonal planar, tetrahedral, linear, and bent geometries – How does hybridization affect bond strength – Shortcut for finding hybridization of any atom

- Formal Charge, Dipoles, Boiling Points, and Curved Arrows – How to calculate the formal charge of an atom – Identifying dipoles – Dipoles as the key to understanding boiling points and reactivity – Dipole moments – Factors that affect boiling points – Four key forces – Curved arrows and how to use them – Three “legal moves” for curved arrows – Examples of curved arrows

- Conformations and Newman Projections – What are conformations – The Newman projection – Staggered vs eclipsed conformations – Identifying syn, anti, gauche interactions – Calculating energies of conformations – Mapping out conformation energies on a graph

- Cycloalkanes – Examples of cyclic alkanes – Geometric isomers – cis and trans – Ring strain of cyclopropane and cyclobutane – Chair conformation of cyclohexane – Drawing Newman projection of a chair – How to do a chair flip – Equatorial and axial positions – A-values – Calculating relative energies of different conformations – Applications in substitution and elimination reactions

- Resonance – What is resonance – Resonance hybrids – How to use curved arrows to interconvert resonance forms – 5 steps to figuring out the most stable resonance form – Applying resonance to understand reactions – pi donors and pi acceptors

- Free Radicals – What is a free radical – 3 key factors that stabilize free radicals – Bond strengths as a guide to radical stability – Showing electron movement with single barbed arrows – Halogenation of alkanes – Recognizing initiation, propagation, termination steps – Identifying free radical initiators – Selectivity of bromine vs. chlorine – Allylic and benzylic bromination – Free radical addition of HBr to alkenes – Calculating selectivity

- Acids and Bases – Introduction to acids and bases – Bronsted and Lewis definitions of acids – Simple examples of acid base reactions – The four key actors in every acid-base reaction – How to define and measure acidity – Ka and pKa – 6 key factors that affect acidity – Charge, electronegatiivty, polarizability, resonance, inductive effects, hybridization – How to tell if an acid-base reaction will be favorable or not using pKa

- Substitution and Elimination – The SN2 mechanism – Steric effects – Polar aprotic solvents and the SN2 – What makes a good leaving group – How the substrate influences reactivity – 4 key factors that affect nucleophilicity – Good, OK and weak nucleophiles – The E2 mechanism – strong bases – Periodic trends for basicity

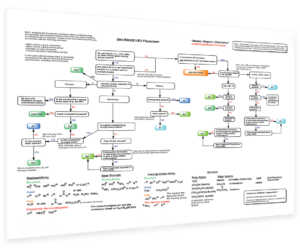
- SN1/SN2/E1/E2 Decision – How to determine if a reaction will proceed via SN1, SN2, E1 or E2? This handy flowchart covers all the possibilities and will help you make the right decision.
- Alkene Reactions – All the key reactions of alkenes on a single page, with notes on their stereochemistry, Markovnikov’s rule, possible rearrangements, and more.

- Alkyne Reactions – Key reactions of alkynes – the reagents, bonds formed/broken, stereochemistry, and more.

- Alcohols and Ethers – Key reactions of alcohols and ethers.

Plus – summary sheets on functional groups, mechanism patterns, reaction energy diagrams, and synthesis.
Join Master Organic Chemistry Membership Today