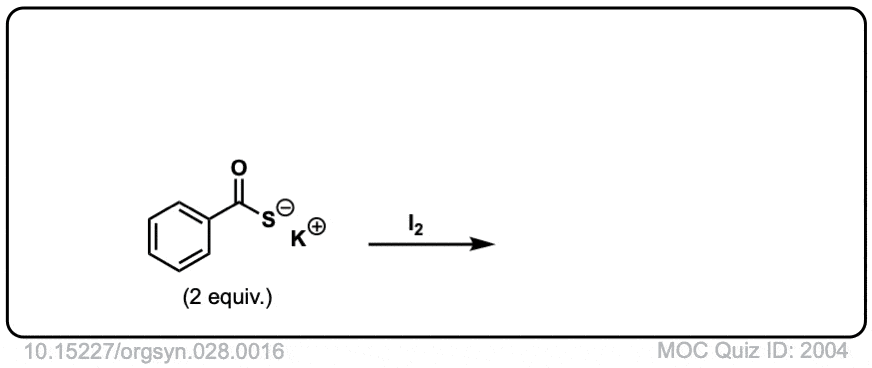
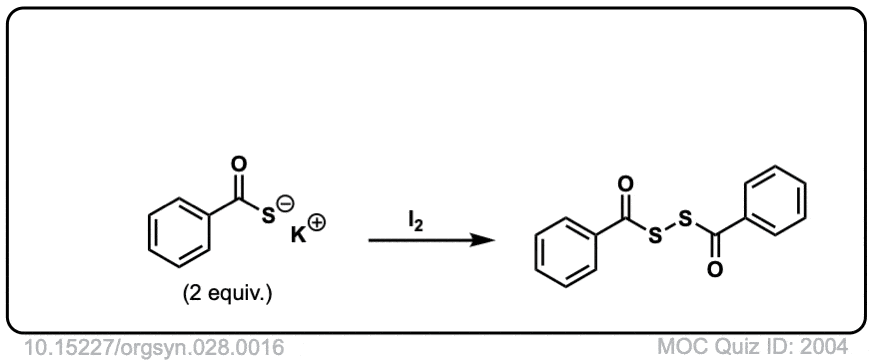
Oxidation of thiols to disulfides
Description: When treated with an oxidizing agent such as iodine or molecular oxygen, thiols can be converted to disulfides.
The rest of this page is available to MOC Members only.
To get access to this page, plus over 2500 quizzes, the Reaction Encyclopedia, Org 1 / Org 2 summary sheets, and flashcards, sign up here for only 30 cents/ day!
Real-Life Example:
Org. Synth. 1948, 28, 16
DOI Link: 10.15227/orgsyn.028.0016
 Click to Flip
Click to Flip
What disulfides would you obtain from oxidation of the following thiols? 3-Methyl-1-butanethiol
Draw two molecules of 3-methyl-butane-1-thiol. Then break both S-H bonds. Then form a new bond between the S and S. That’s your molecule.