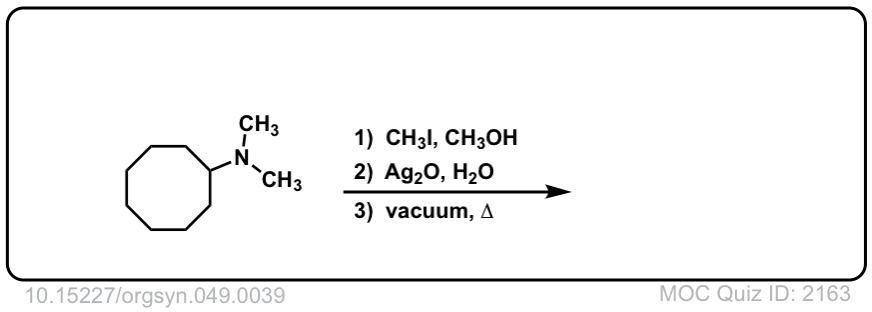
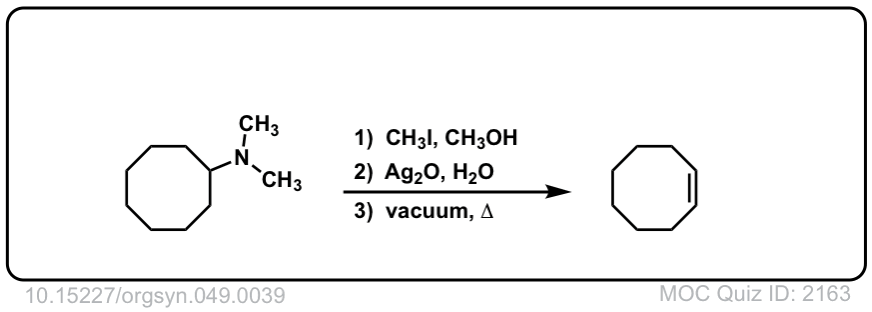
Hofmann elimination of alkylammonium salts to give alkenes
Description: Tetra-alkyl ammonium salts undergo elimination to form the least substituted alkene. This is called the Hofman elimination.
The rest of this page is available to MOC Members only.
To get access to this page, plus over 2500 quizzes, the Reaction Encyclopedia, Org 1 / Org 2 summary sheets, and flashcards, sign up here for only 30 cents/ day!
Real-Life Example:
Org. Synth. 1969, 49, 39
DOI Link: 10.15227/orgsyn.049.0039
 Click to Flip
Click to Flip
Hey James; love the content thus far. I believe the Newman projection on the left may be missing a carbon from the main alkyl chain (4 shown when there should be 5). I don’t know if that was intentional, but regardless I just want to say thanks for making this website.
Much love.
Shoot. You are correct Jackson. I’ll put that on the fix list!
Fixed.
Just curious. What would happen if we tried reacting Tetramethyl Ammonium with a mild base (like AgOH). Since there is no beta Hydrogen to abstract, Hofmann elimination isn’t feasible so what would be the products?
Probably nothing would happen. You might get a little bit of methanol and NMe3 via substitution (SN2).
That’s one f in Hofmann ….
Thank you.
I appreciate your well presented information. I just wanted to ask whether you meant, under bonds broken, C1-H and not C2-H. That is in the first (i.e, general) equation. Later on, under mechanism, the information looks correct.
Thank you.
Thanks for the spot! Fixed.