The Gabriel synthesis of amines
Description: The Gabriel synthesis is a method for making primary amines through an SN2 reaction of a phthalimide with an alkyl halide followed by cleavage with hydrazine (NH2NH2).
The rest of this page is available to MOC Members only.
To get access to this page, plus over 2500 quizzes, the Reaction Encyclopedia, Org 1 / Org 2 summary sheets, and flashcards, sign up here for only 30 cents/ day!
Real-Life Example:
Org. Synth. 1947, 27, 12
DOI Link: 10.15227/orgsyn.027.0012
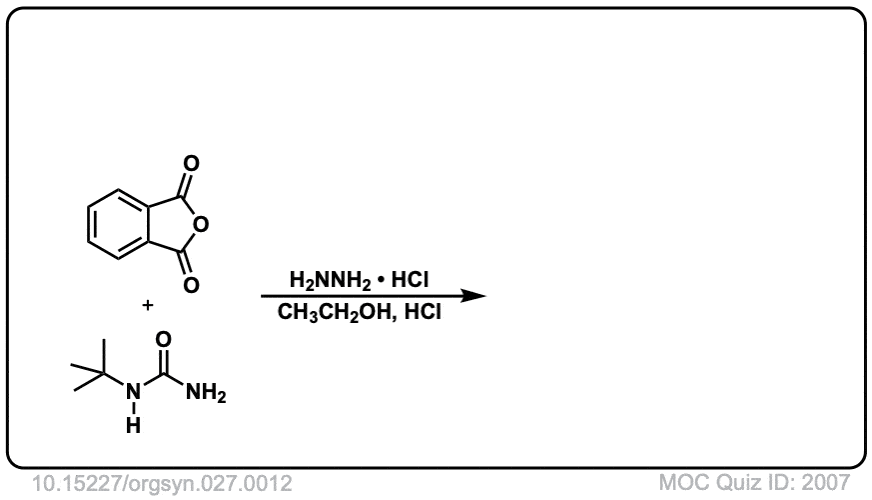 Click to Flip
Click to Flip
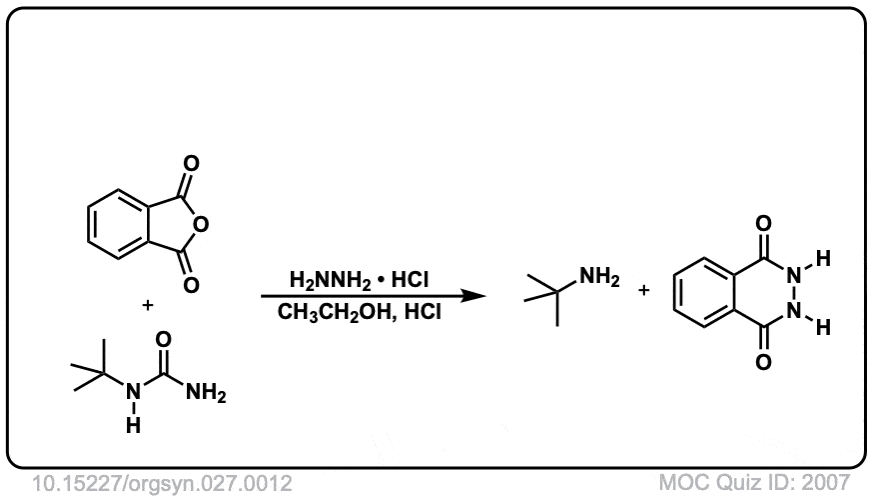
Hi, in step 6 and 7 you are missing the letter R.
Fixed, thank you!
Hi,
When would you use Gabriel vs Azide to make a primary amine in synthesis?
Great question! For uncomplicated molecules without a lot of different functional groups, they both work.
From a practical standpoint, N3- tends to get more use.
N3- tends to be a better nucleophile (faster rxn) than potassium phthalimide. Also, reduction of N3 to NH2 can be done many different ways (Pd-C/H2, LiAlH4, etc) and only blows off N2, whereas cleavage of the phthalimide necessitates getting rid of the byproduct.
For your purposes though – either one is probably fine!
Hi James,
Which functional groups will not tolerate either/both of these transformations (i.e. azide SN2 & Gabriel rxn)?
Thanks!
That’s kind of a broad question. With the Gabriel you’d want to avoid any unprotected aldehydes/ketones for a variety of reasons, particularly the deprotection step. That’s just a start.