Addition of Grignard reagents to esters to give tertiary alcohols
Description: Grignard reagents add twice to esters, giving tertiary alcohols (after addition of acid)
The rest of this page is available to MOC Members only.
To get access to this page, plus over 2500 quizzes, the Reaction Encyclopedia, Org 1 / Org 2 summary sheets, and flashcards, sign up here for only 30 cents/ day!
Real-World Examples:
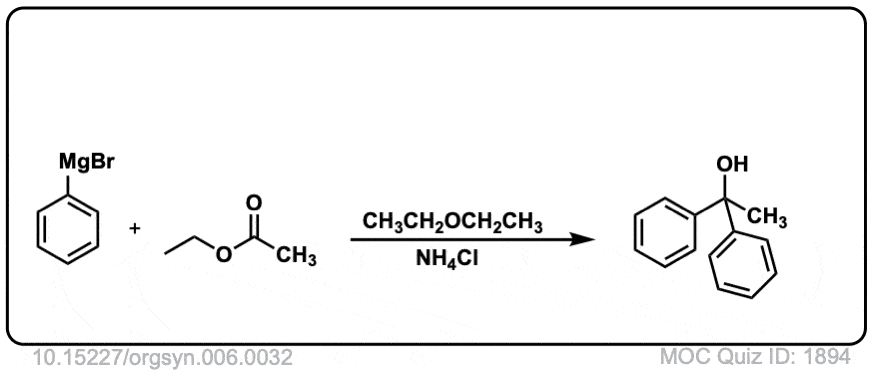
Org. Synth. 1926, 6, 32
DOI Link: 10.15227/orgsyn.006.0032
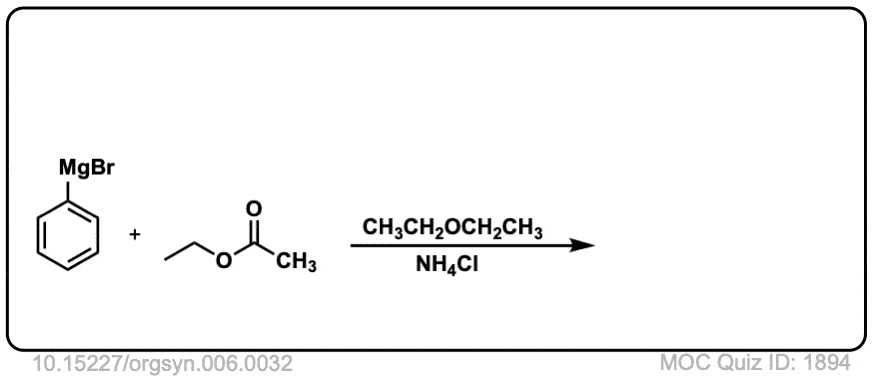 Click to Flip
Click to Flip
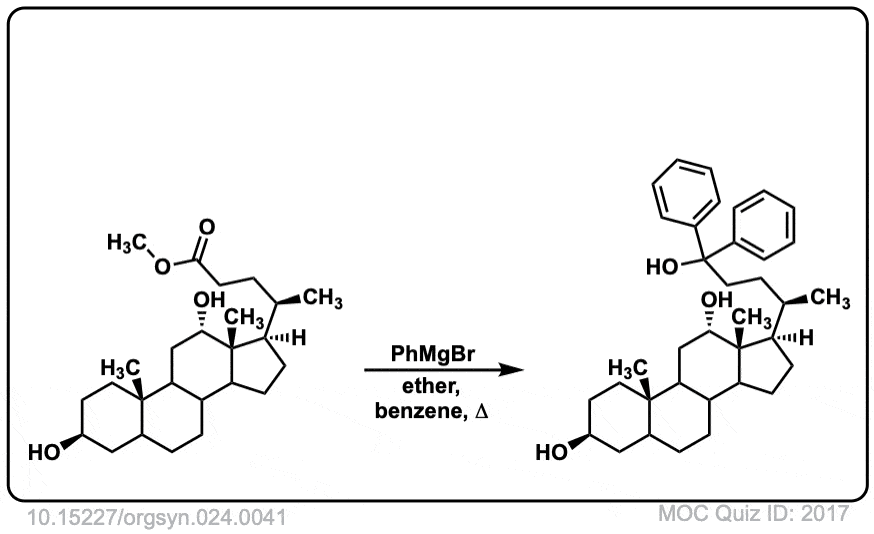
Org. Synth. 1944, 24, 41
DOI Link: 10.15227/orgsyn.024.0041
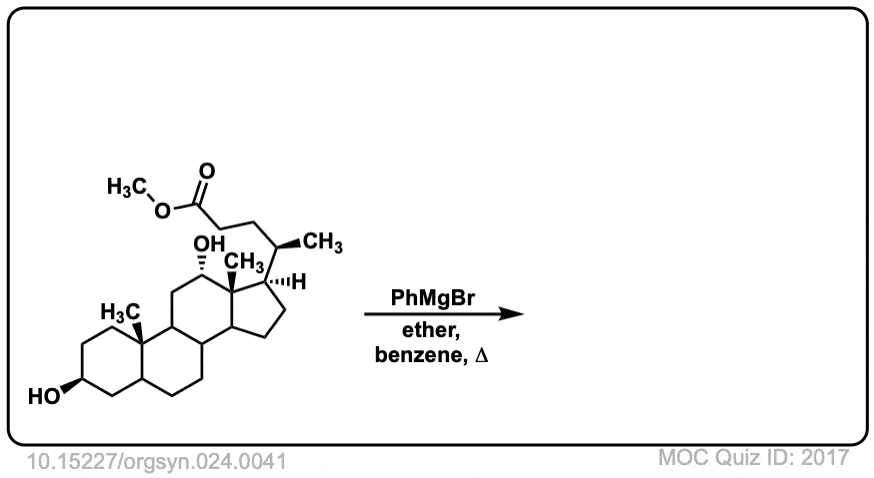 Click to Flip
Click to Flip
Org. Synth. 1943, 23, 98
DOI Link: 10.15227/orgsyn.023.0098
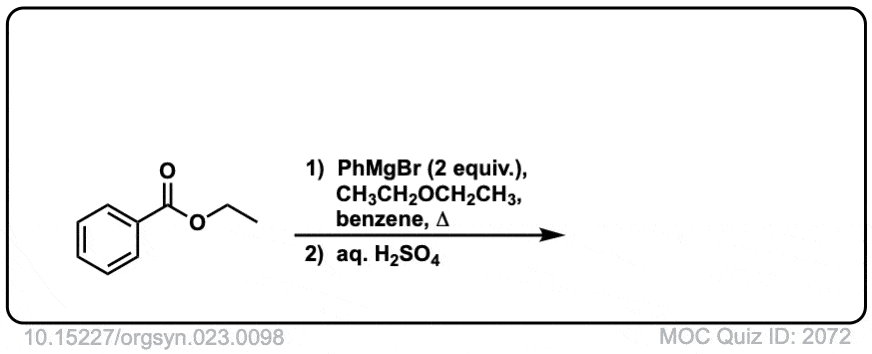 Click to Flip
Click to Flip
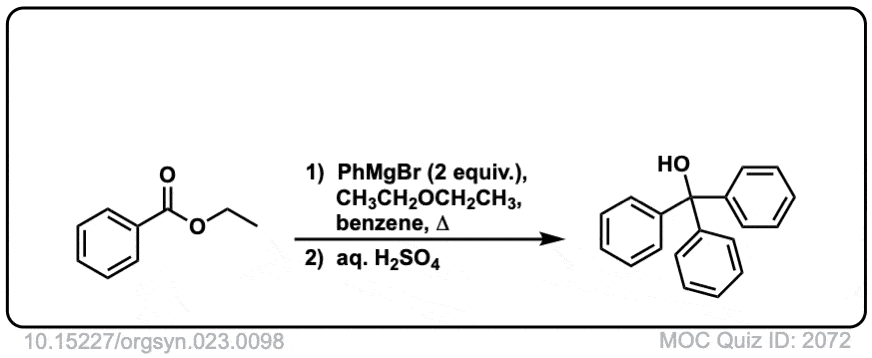
Org. Synth. 1958, 38, 41
DOI Link: 10.15227/orgsyn.038.0041
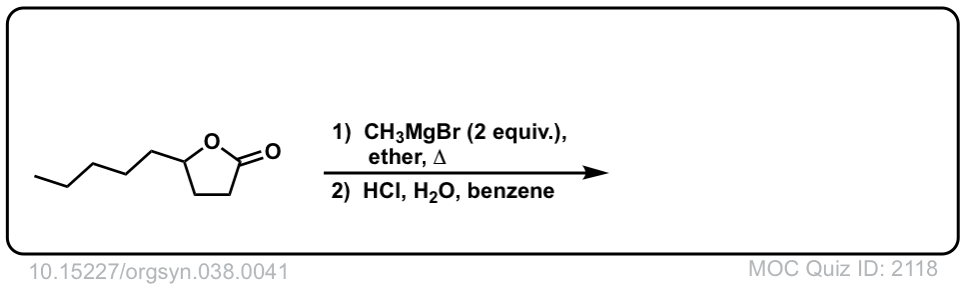 Click to Flip
Click to Flip
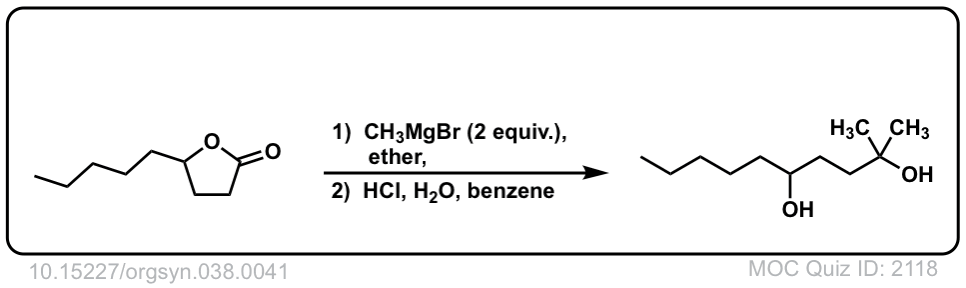
In the third example shouldn’t the diol go through dehydration and become a cyclic ether
Yeah, I think you are right. The product can form stable carbocation in the more substituted carbon and then cyclic reaction.
Good point. Instructors differ on how to handle the quench step. To some, H+ is “too harsh”, resulting in precisely the result you describe. To others, “H+” is fine. I should have written, “mild acid”.