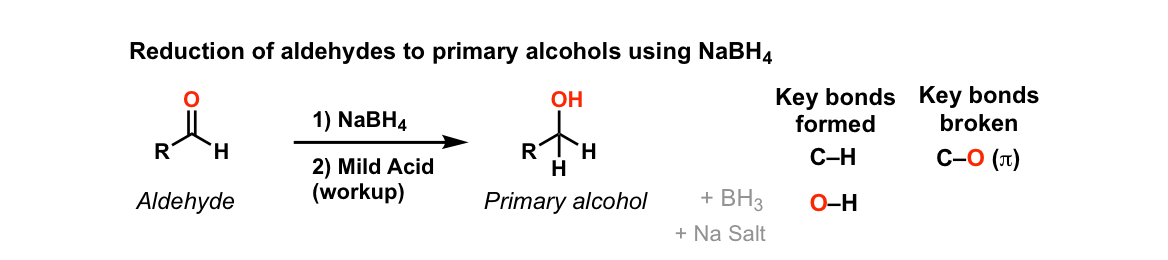
Addition of NaBH4 to aldehydes to give primary alcohols
Description: Addition of sodium borohydride (NaBH4) to aldehydes gives primary alcohols (after adding acid)
Examples:
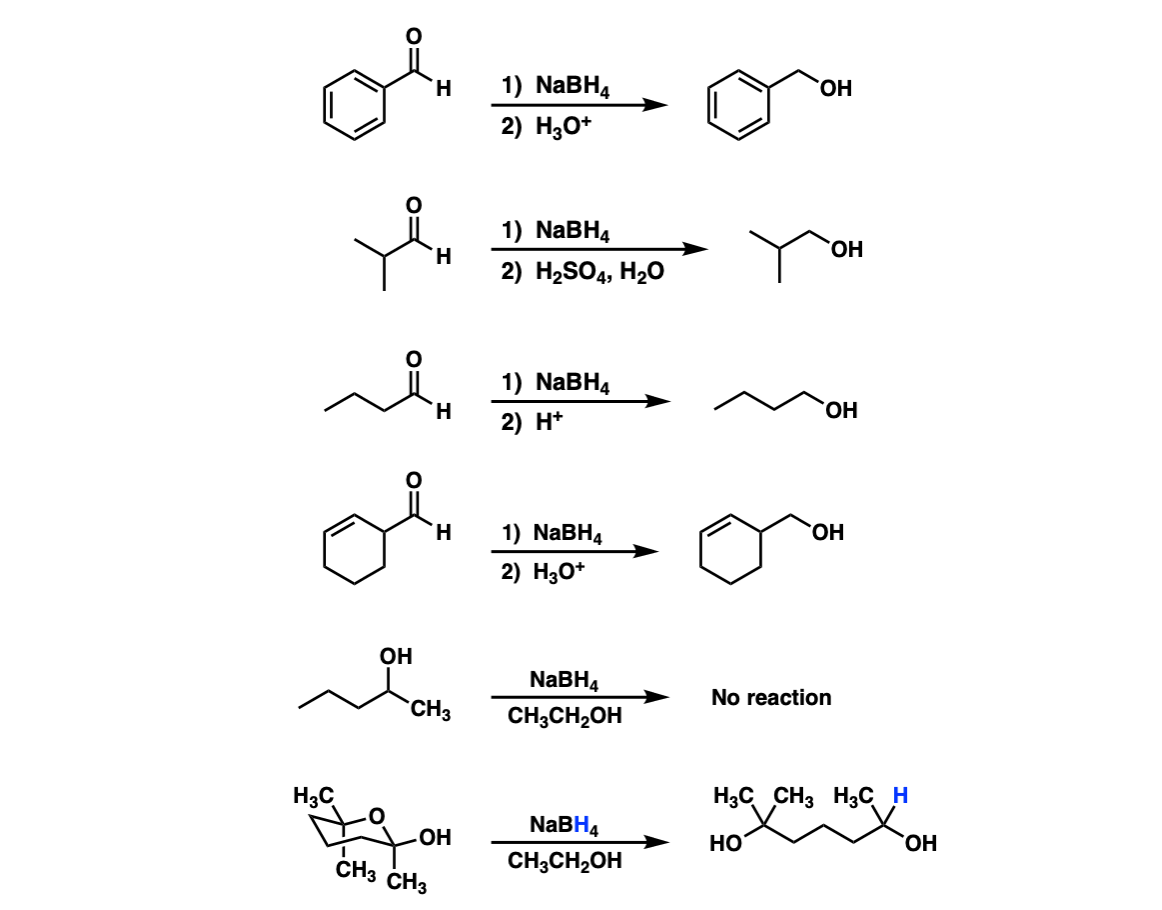
Notes: Lots of different acids can be used in the last step. It’s not important which specific acid is used, just that something is present that can form the alcohol.
In the fifth example there is no aldehyde present so no reduction can occur. The sixth example is a hemiacetal that opens into an aldehyde and is then reduced by NaBH4.
For another example of this see Ring-Chain Tautomerism
Mechanism:
NaBH4 is a source of hydride (H-) and the reaction begins with the addition of hydride to the carbonyl to the aldehyde (Step 1, arrows A and B). Upon addition of acid, the oxygen is protonated (Step 2, arrows C and D) to give the neutral primary alcohol.
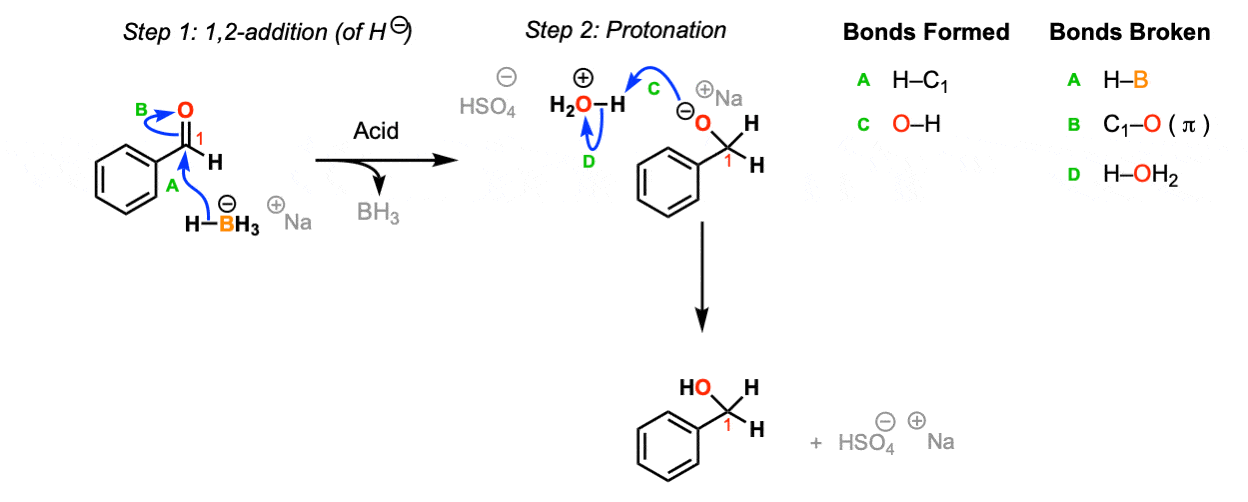
Notes: The choice of H2O / H2SO4 as acid isn’t crucial – this is just an example. Any source of proton (including water) will do.
Quiz Yourself!
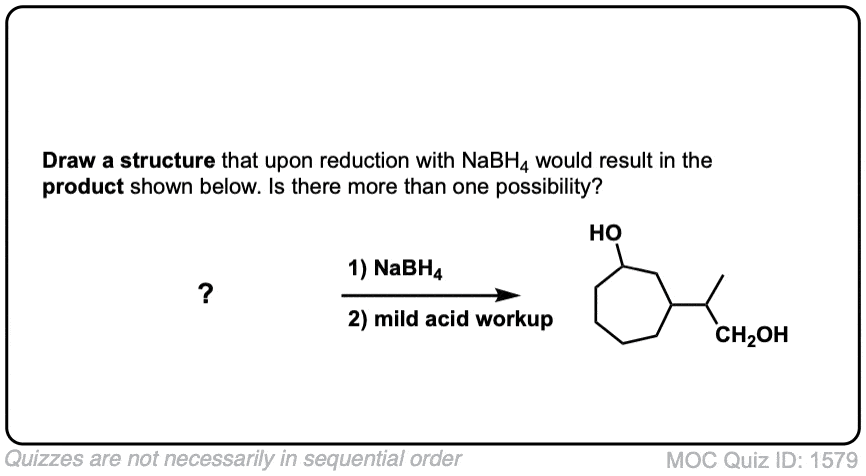 Click to Flip
Click to Flip
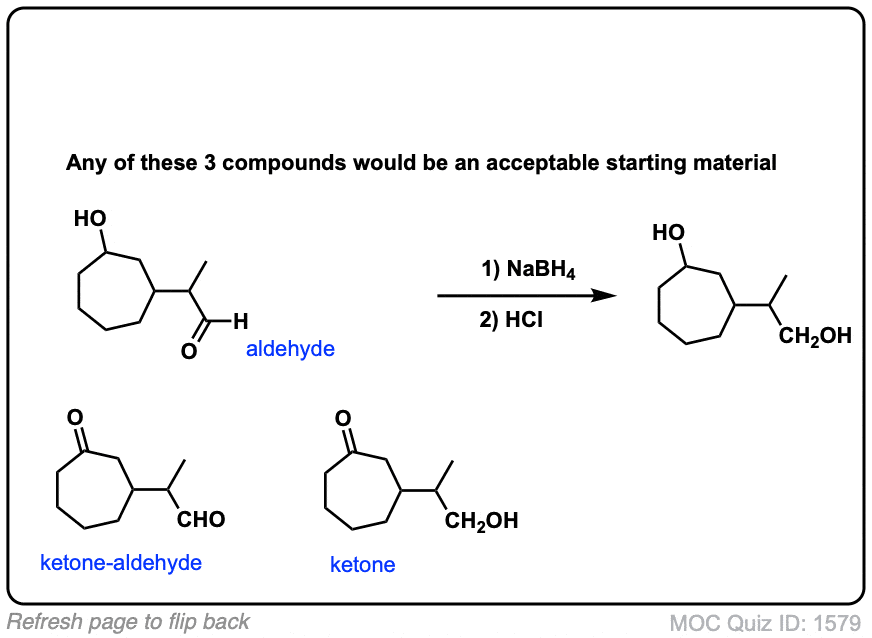
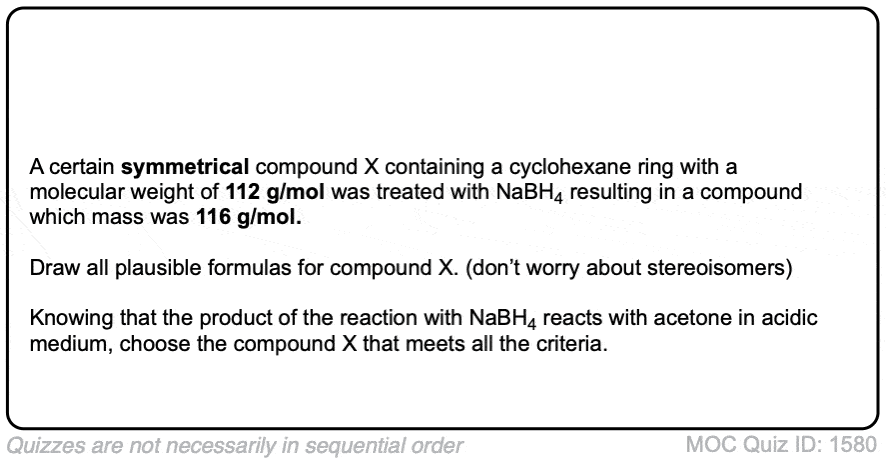 Click to Flip
Click to Flip
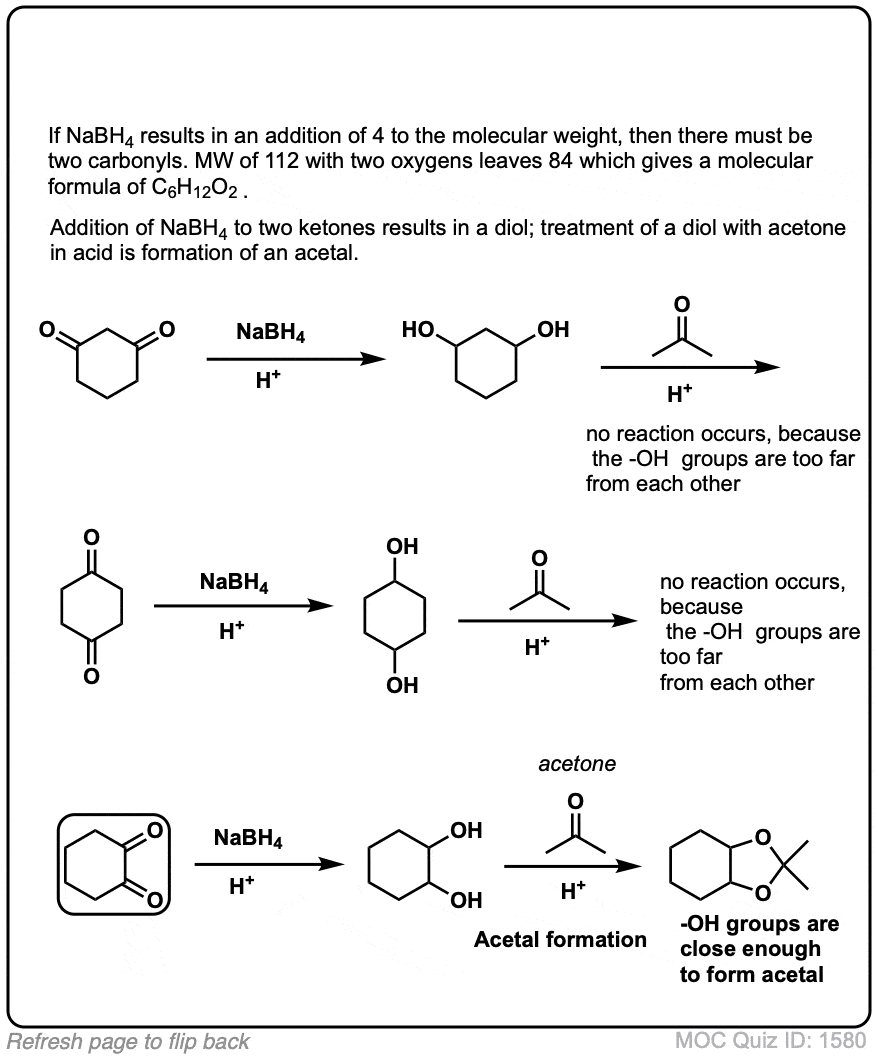
(Advanced) References and Further Reading:
- First example
Brown, H.C.; Wheeler, O.H.; Ichikawa, K.
Tetrahedron 1:214 (1957)
DOI: 10.1016/0040-4020(57)88041-7
Early paper by Nobel Laureate H. C. Brown describing the reactivities of simple aldehydes and ketones to reduction by NaBH4, in which it is shown that aldehydes are more reactive than ketones to nucleophilic reactions. - Mechanistic studies
Brown, H. C.; Ichikawa, K.
Tetrahedron 1:221 (1957)
DOI: 10.1016/0040-4020(57)88042-9
This paper and the above are both mechanistic studies on the reduction of carbonyls – this paper investigates the effect of ring size on the reduction of cyclic ketones (e.g. reduction of cyclobutanone vs. cyclopentanone, cyclohexanone, etc.). - Reference To An Experimental Procedure
Antonio Bermejo Gómez, Nanna Ahlsten, Ana E. Platero-Prats and Belén Martín-Matute
Org. Synth. 2014, 91, 185
DOI: 10.15227/orgsyn.091.0185
The first step in this procedure uses NaBH4 to reduce a cinnamyl ketone to the alcohol.
What will happen when Alpha halo carbonyl compound treated with nabh4 and meoh 25 c temperature
Darzens.
Well, how does ethers react with NaBH4? Does any reaction happen at all?
No reaction at all occurs with ethers and NaBH4.
https://youtu.be/R_g088IYjGE
spray chrome
If you have a silver salt (Ag+) in solution and add NaBH4, you’re going to end up with metallic silver. Beyond that, I don’t know what to tell you.
Hi, I am using spray mirror silver, ammonia, sodium hydroxide and in my second solution of boric acid and sodium borohydride. Can I introduce formalin or other material to achieve better reaction and increase the quality of mirror quality? do .
Matt used hydrazine hydrate and in the old days I also used glucose and formalin, but now sodium hydrochloride is rapidly increasing.
I have no idea what you are trying to do.
What is the reaction of formaldehyde with NaBH4/H20?Reaction?
Hi Sherri . A new C-H bond would form, and the C-O pi bond would break. After adding water (or mild acid) a new O-H bond would form. That would provide methanol, CH3OH.
Hi James
What happens if a group like C=NH is reduced using NaBH4.
Does NaBH4 reduces every polar π bonded group?
Imines can be reduced with NaBH4 but usually as the iminium ion (i.e. the conjugate acid of imines). This is the key process in “reductive amination”. NaBH4 certainly does NOT reduce very polar pi bonded group – it does not always reduce C=O bonds, for example. Esters and amides are generally not reduced by NaBH4 for example.
what if a base is used instead of an acid??
Eg. NaOH
Why would you do that? You’re just going to get the alkoxide.
why would nabh4 be more reactive than nabh3cn ?
NaBH3CN is a poorer nucleophile because CN is an electron withdrawing group, and removing electron density will make the B-H bond a poorer nucleophile.
what would be the expected products if we use NaBD4/h2o or NaBD4/D2o??
NaBD4 will form a C-D bond instead of a C-H bond. If you quench with H2O you will form an O-H bond. If you quench with D2O you will form an O-D bond.
Is KBH4 a worse reducing agent as NaBH4 for this reaction or are the about the same?
Hi – I would expect that KBH4 is slightly worse, as K+ is not as good a Lewis acid as Na+.
LiBH4 , for example, is a stronger reducing agent than NaBH4. LiBH4 can reduce esters whereas NaBH4 cannot.
Hope that helps!
James
What if NaBH4 is in aqueous NaOH (or other base)? Will it still reduce ketones/aldehydes?
Why would you want to do that?
One concern is that you’d form either the enolate or the hydrate of the ketone/aldehyde, in which case the reduction would not occur.
Does NaBH4 lead to production of diols in conversion of cyclo butane to butan-1,4-diol
No, NaBH4 would not produce a diol from cyclobutane. Breaking the C-C bond of cyclobutane requires more than that.
Can NaBH4 and LiAlH4 cleave C=C ????????
Only in conjugated systems, e.g. cyclopent-2-enone
If the solvent is Ethanol and acid does not occur
Methanol is a common solvent for this reaction. Adding acid is not, unless you count Lewis acids like CeCl3
Can NaBH4 reduce alkene with electronwithdrawing like NO2 ?? And CO too?
Yes, this is known as “conjugate reduction”. Alkenes adjacent to carbonyls or nitro groups will be reduced to alkanes. However, in the case of ketones, if a strong Lewis acid like CeCl3 is used, reduction with NaBH4 occurs just at the carbonyl and leaves the alkene intact. This is known as the Luche reduction. https://en.wikipedia.org/wiki/Luche_reduction
What is the result of reaction involving D-glyceraldehyde and NaBH4? Would it be glycerol?
Yes, it certainly would be.
I just wanted to ask if there is any colour change that occurs during the reduction of the aldehydes to alcohols by NaBH4
Thanks in advance.
Color changes are not a very diagnostic criteria for reactions. It’s very common to see a pale yellow colour, but that’s not unusual for a large number of other reactions.
how can the colour formation be prevented or can we decolourize it
Color is not important for this process. Why is it important to you?
color is important ,because it gives the impression that the material is contaminate
dear Zia,
if you have color impurity(if you are very sure) then you can treat your compound with charcoal. And this mostly works with liquid compounds. You can add a pinch of charcoal in your compound and let it stirring for some 10 minutes.
thanks
i used activated carbon ,the orange color remained unchanged. would you tell me if there is any substance that could decolorise primary alcohol made by reacting sodium boro hydride on aldehyde
can we use for ring reduction using sodium borohydride like pyridine and pyrrole are other heterocyclic answer me
No, it is not a strong enough reductant.
Hey
Got a question regarding reduction of P-nitrobenzealdehyde to p-nitrobenzyl alcohol. We mixed sodium hydroxide and potassium borohydride and can’t figure out how the mechanism takes place. Does sodium hydroxide have anything to do with it, or is it only the aldehyd + potassium?
Thanks
I’m not sure why you added NaOH with KBH4. By the way why did you use KBH4 – you usually have to make it, and NaBH4 is much cheaper?
The mechanism for reduction with a borohydride is simply that the B-H bond breaks, donating two electrons to form a new bond with the carbonyl carbon, and the C-O pi bond breaks. You end up with an alkoxide, which is then protonated to give the alcohol.
what if we use NaBD⁴ in H²0² with OH·-· to reduce propene ? The answer is ⛪ CH³CHDCH²CH²OH.. please give the mechanism
Hey, what happens when there is an excess of NaBH4?
Nothing bad should happen. Once the aldehyde is reduced to the alcohol, there’s nothing else that can happen (assuming you just have one aldehyde).
Could you explain why in some reaction condition there were also iodine added?
I’m wonder whether its goes through IBH2 reactant or this author(http://totallymechanistic.wordpress.com/2006/12/11/sodium-borohydrideiodine-reduction/) states that the reductant is the borane (BH3) which is generated in situ. Yet some people think it’s the BH3-THF complex being reductant, and then I’m not sure what’s the Iodine for.
Thank you!!
Iodine is used to form BH3 in situ from NaBH4 . It’s used, for instance, in the reduction of carboxylic acids to alcohols.
Hi James,
It seems like the BH4 in step 1 is nucleophilic, and the H-B bond is moving (Electrons and H) to the C=O, which is why the C-O bond ‘jumps’ up. Is this not a substitution reaction? Or perhaps my definition of an Addition reaction is a bit narrow?
There is a problem in my book I am having a small issue with. It states “Draw the products formed when CH3COCH2CH2CH=CH2 is treated with NaBH4 (excess) in CH3OH”. I understand that NaBH4 selectively reduces C=O while leaving C=C inert, but what protonates the oxygen in the final step to give the alcohol? Does NaBH4 do that because it is in excess, or does methanol? I don’t 100% understand methanol’s role in the reaction, just that it is typically used with NaBH4.
in prescence of meoh, the MeOH acts as a proton source and also there will be formation of methoxy borohydride (mono,di,tri) which has higher susceptibility of donating the hydride. because of these two reasons MeOH or EtOH are often used as co-solvents in NaBH4 reduction
True, but actually methoxyborohydrides are not as nucleophilic as sodium borohydride itself.
Yes,ofcourse Methanol would get protonate the oxygen in final step because it is present in excess and it release it’s H-atom easily under given condition.
Thank you, very helpful and complete, answered every question!