Addition of Organolithiums To Aldehydes and Ketones
Description: Organolithium reagents (RLi) will add to aldehydes and ketones to give alcohols, much like Grignard reagents.
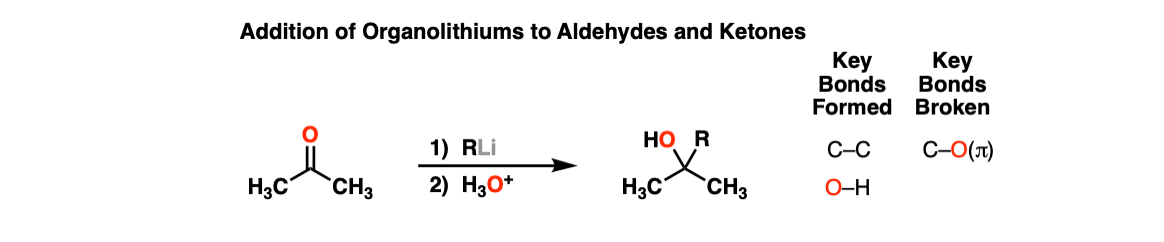
Notes: H3O+ here just denotes “acidic workup”, a brief treatment of the reaction mixture with acid in order to neutralize any strong bases.
In practice, one can use a dilute solution of acid like 1 M H2SO4 or KHSO4 or in cases where a very mild acid is required, a solution of NH4Cl (ammonium chloride) but one is rarely asked to provide such details.
Examples:
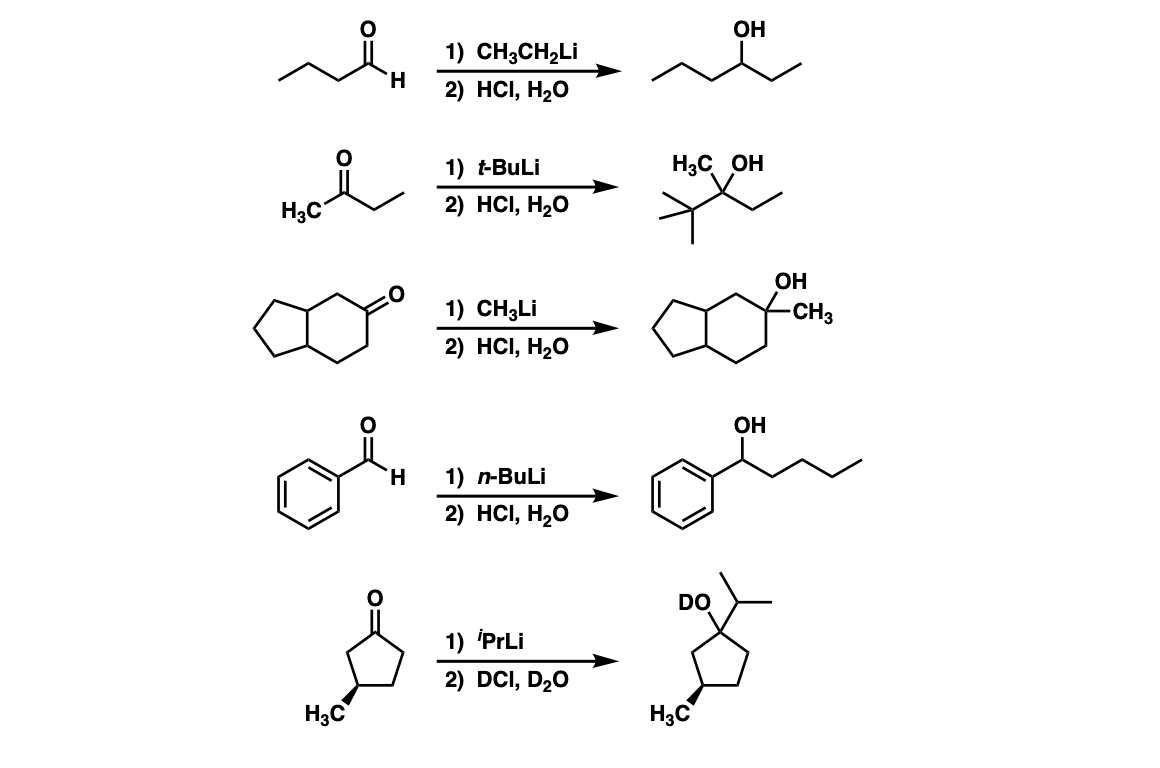
Notes: Addition of an organolithium to an aldehyde will result in a secondary alcohol (Examples 1 and 4). Addition of an organolithium to a ketone will give a tertiary alcohol (Examples 2 and 3).
Note that if a deuterated acid is used in the workup (D = deuterium, the heavy isotope of hydrogen) then the resulting alcohol will have a deuterium (D) attached to oxygen instead of a proton (H) (example 5).
Mechanism:
The first step of the reaction is addition of the organolithium to the aldehyde or ketone (Step 1, arrows A and B). This is the end of the reaction until workup with mild acid, whereupon the negatively charged oxygen (“alkoxide”) is protonated with mild acid (Step 2, arrows C and D) to give the final alcohol product.
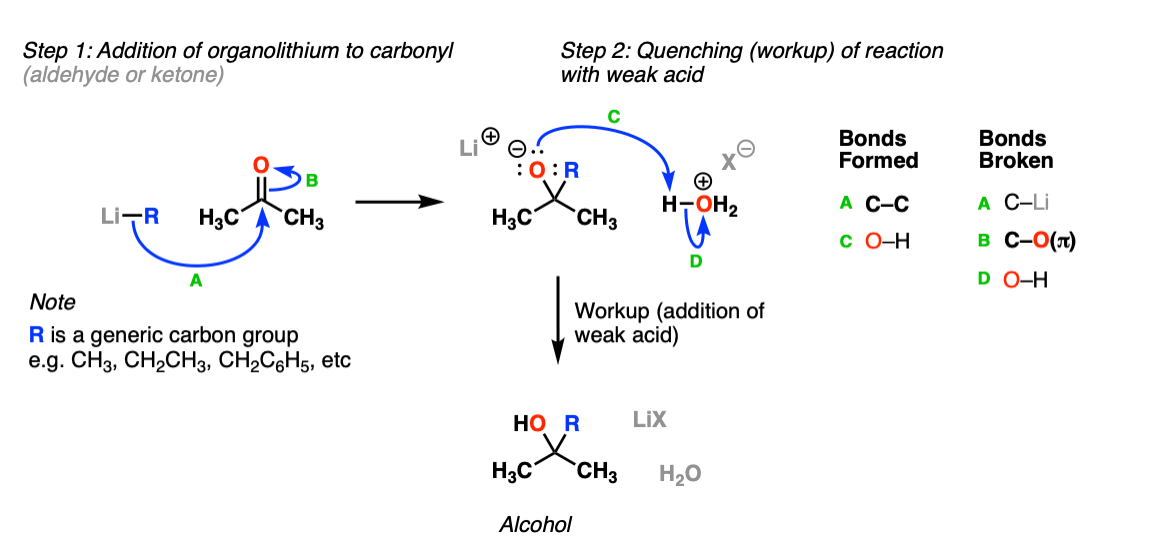
Notes: The specific acid to be used is not crucial here; H3O+ is a pretty generic term for aqueous acid.