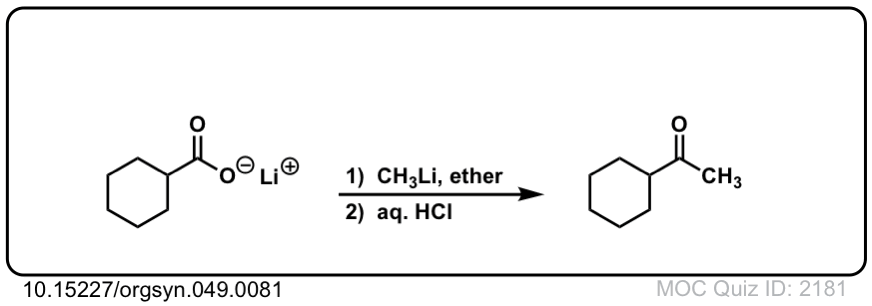
Addition of Organolithiums to Carboxylic Acids
Overview: Adding two equivalents of an organolithium reagent to a carboxylic acid will result in a new ketone.
Notes: This reaction does not work with Grignard reagents, only organolithium reagents. The purpose of H3O+ at the end is to protonate the negatively charged oxygen.
Examples:
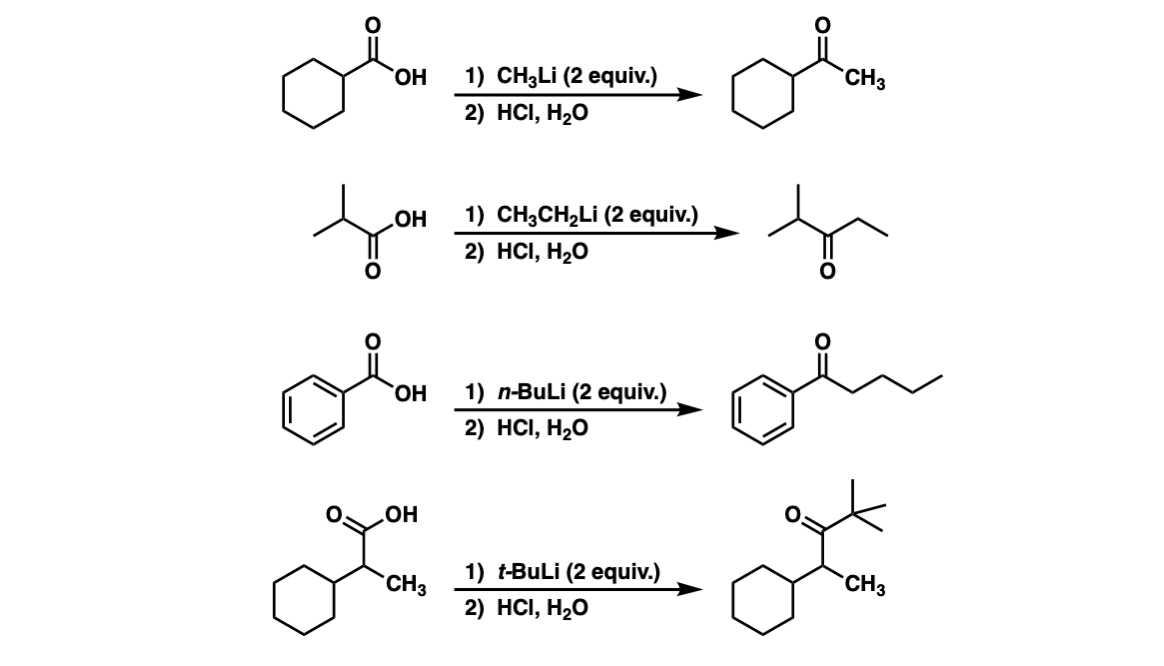
Notes: Each of these four examples show the addition of two equivalents of an organolithium reagent to a carboxylic acid with the end result of producing a ketone. The purpose of HCl and H2O at the end is to form H3O+.
Mechanism:
The first step is deprotonation of the carboxylic acid by the strongly basic organolithium species (Step 1, arrows A and B). This is followed by addition of a second equivalent of organolithium reagent to the carbonyl carbon (Step 2, arrows C and D). The resulting di-anion is stable in solution until acid is added (workup), resulting in a hydrate (Step 3). The hydrate is protonated further with acid (Step 4, arrows E and F) followed by elimination of water (Step 5, arrows G and H) to give the protonated ketone, which is then deprotonated by water (Step 6, arrows I and J) to provide the ketonoe product.
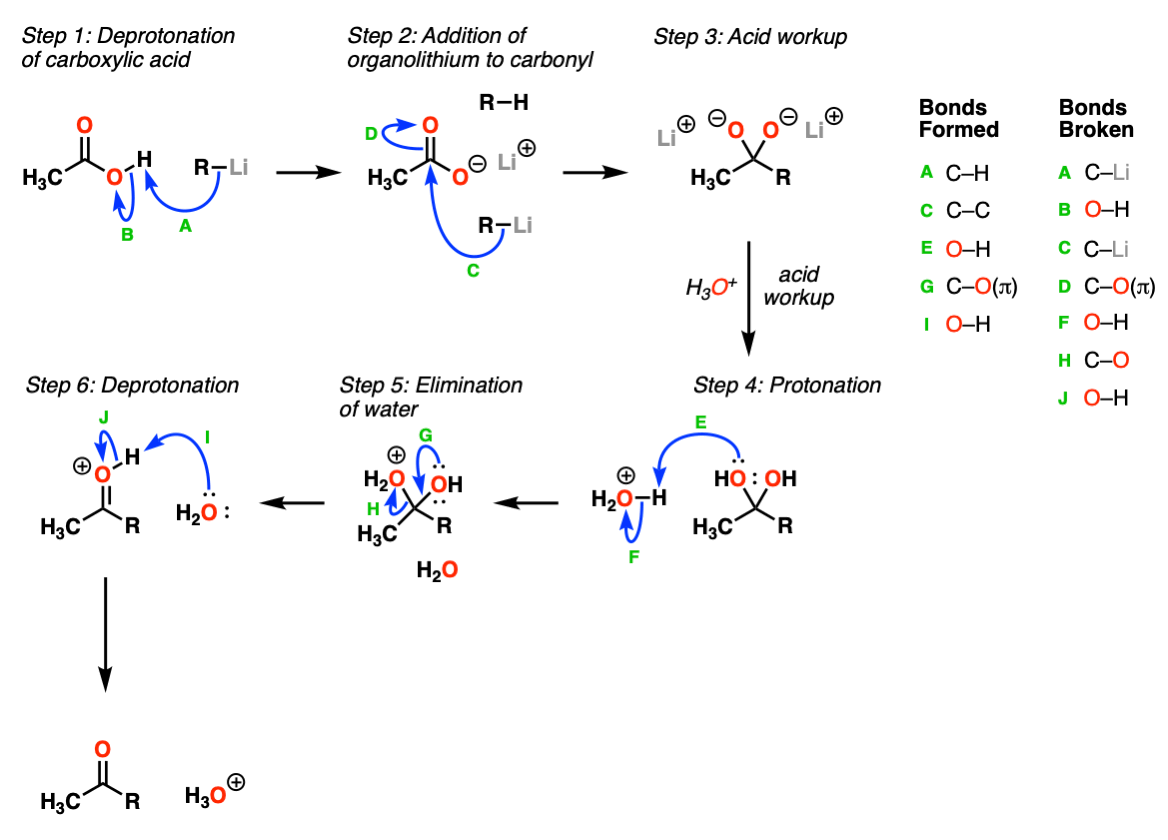
Notes: The key reaction here is the addition of the organolithium reagent to the deprotonated carboxylic acid (Step 2, arrows C and D). This forms a stable tetrahedral intermediate which sits around until acid is added.
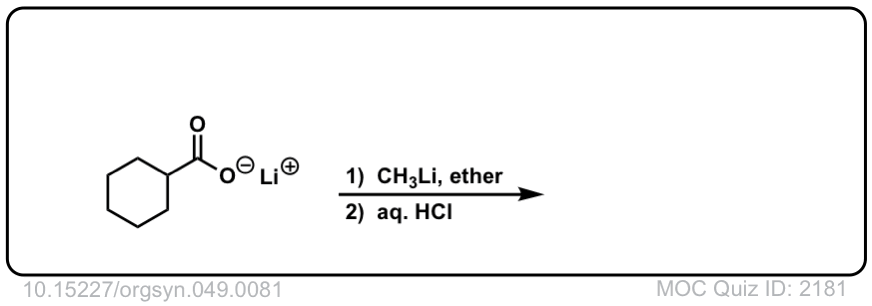
Real-Life Example:
Org. Synth. 1969, 49, 81
DOI Link: 10.15227/orgsyn.049.0081
 Click to Flip
Click to Flip