Amine Protection and Deprotection
Description: Amines can be protected as carbamate groups using reagents like Boc2O, CBzCl, and FMOC-Cl. These carbamates can be removed using acid (e.g. trifluoroacetic acid for Boc), catalytic hydrogenation (Pd-C, H2 for the CBz group) or basic conditions (piperidine for FMOC) respectively.
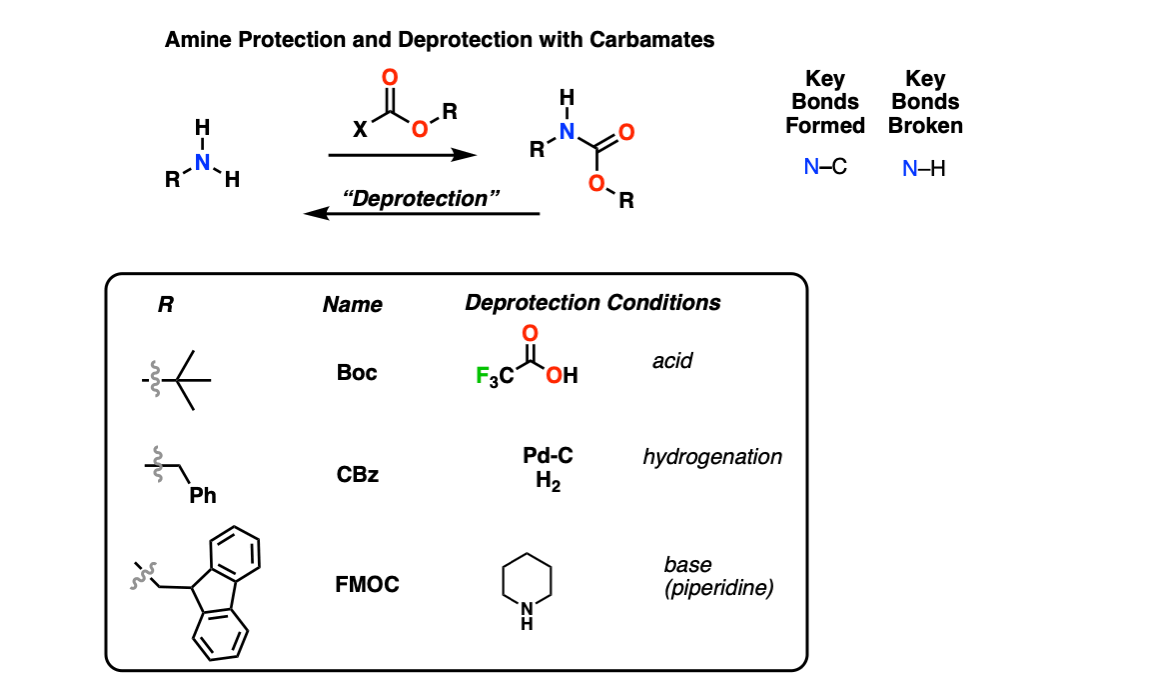
Notes: Amine protection is particularly important for peptide synthesis.
Examples:
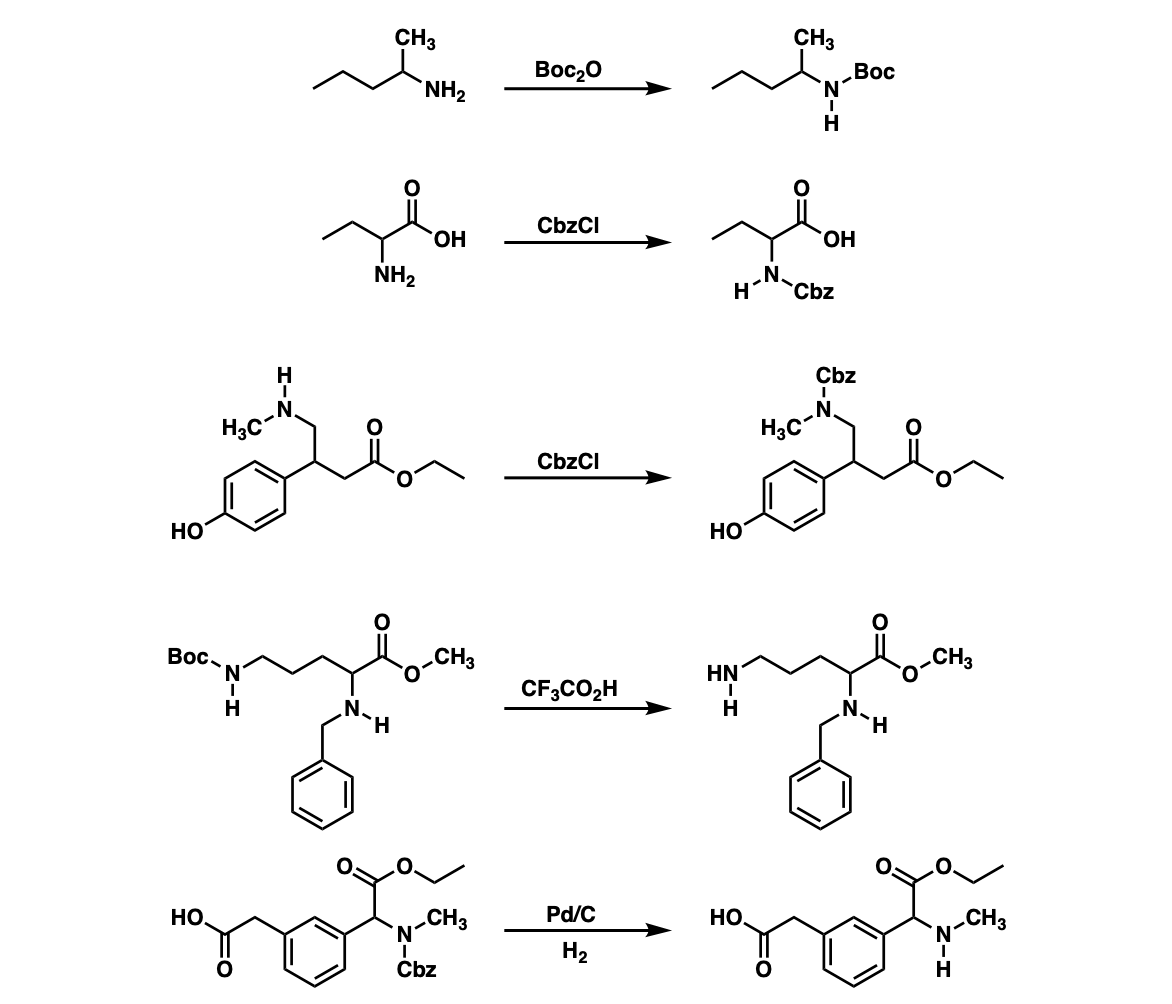
Notes: Example 1 shows a Boc protection. Examples 2 and 3 show CBz protection. Example 4 shows a Boc deprotection of the amine under acidic conditions which leaves the CBz protected amine unaffected.
Example 5 shows removal of the CBz protecting group from the amine using catalytic hydrogenation.
Mechanism: Protection of amine using Boc2O (Di-t-butyl dicarbonate).
In the first step, the amine attacks the carbonyl carbon of the carbonate (Step 1, arrows A and B) which forms a new tetrahedral intermediate. Elimination of oxygen (Step 2, arrows C and D) results in loss of a carbonate which itself can act as a base, or spontaneously decarboxylate (lose CO2) to give t-butoxide, which then neutralizes the protonated carbamate.
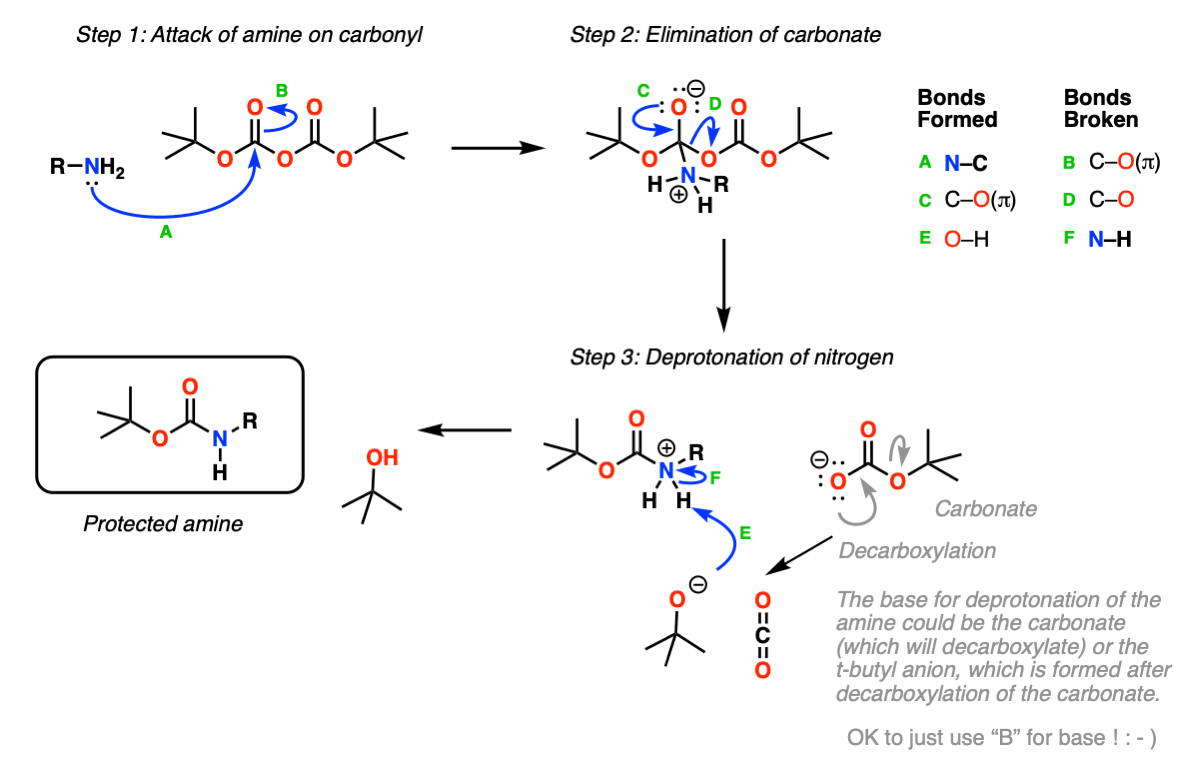
This probably oversimplifies things a bit; a proton transfer from the nitrogen to oxygen after Step 1 is also likely and would eventually give the same result, we’re going for simplicity here.
Deprotection: Protonation of the carbamate oxygen with a strong acid such as trifluoroacetic acid (TFA) (Step 1, arrows A and B) then results in loss of a t-butyl carbocation (Step 2, arrows C and D). The resulting “carbamic acid” (a nitrogen attached to CO2H) undergoes proton transfer (Step 3, arrows E and F) followed by decarboxylation (Step 4, arrows G and H) to give the neutral, deprotected amine.
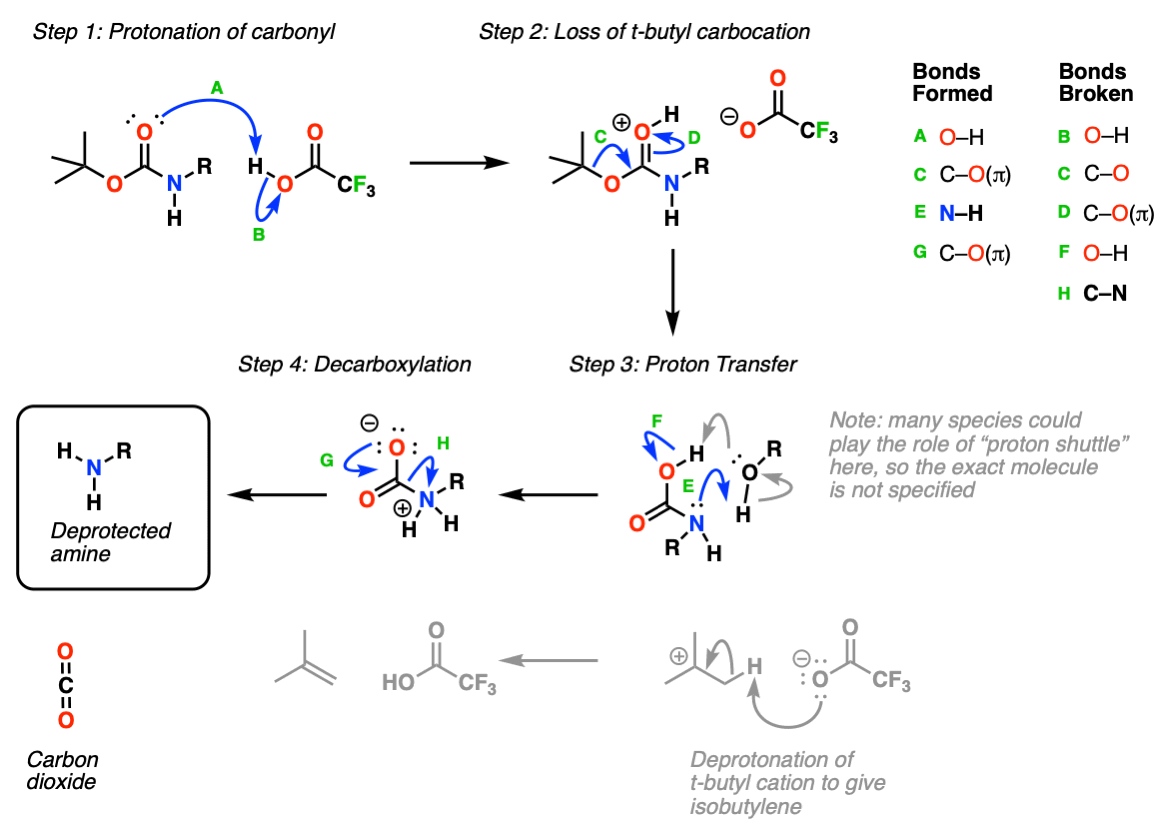
Notes: In practice this is one of the simplest reactions to perform in all of organic chemistry. Simply add “neat” TFA to a dilute (~ 0.2 M) solution of your protected amine, and pop! off comes the carbamate. Watching the CO2 bubble off is very satisfying.
Alternatively, heating the crap out of the protected amine (ca. 180 degrees C under vacuum) will also result in loss of the protecting group.
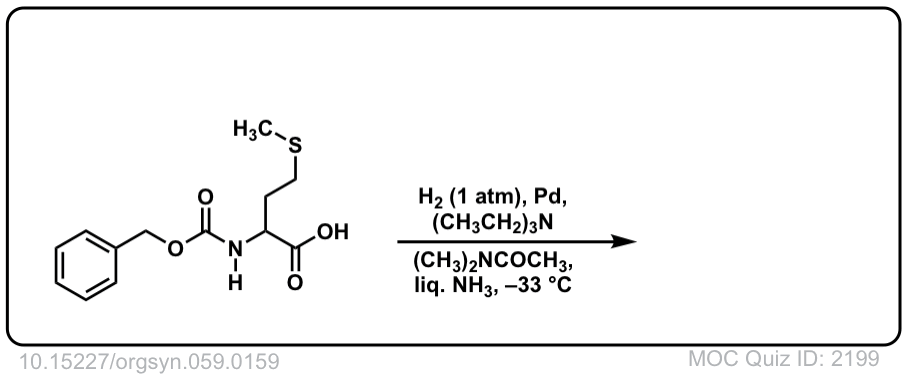
Real-Life Example:
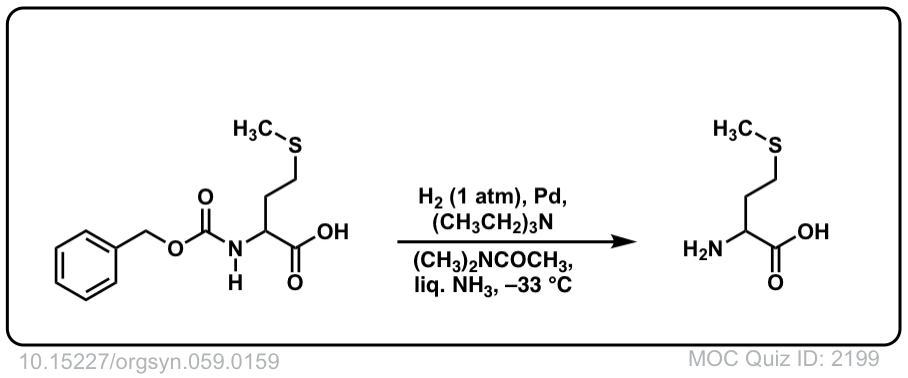
Org. Synth. 1979, 59, 159
DOI Link: 10.15227/orgsyn.059.0159
 Click to Flip
Click to Flip