Basic hydrolysis of esters (saponification)
Description: Esters can be converted to carboxylic acids through treatment with strong base.
The rest of this page is available to MOC Members only.
To get access to this page, plus over 2500 quizzes, the Reaction Encyclopedia, Org 1 / Org 2 summary sheets, and flashcards, sign up here for only 30 cents/ day!
Real-Life Examples:
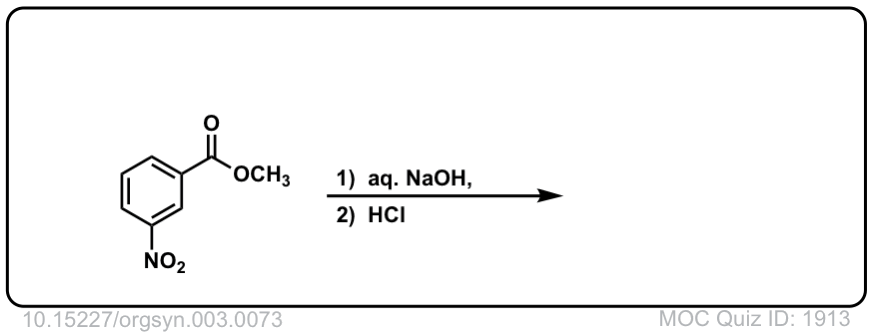
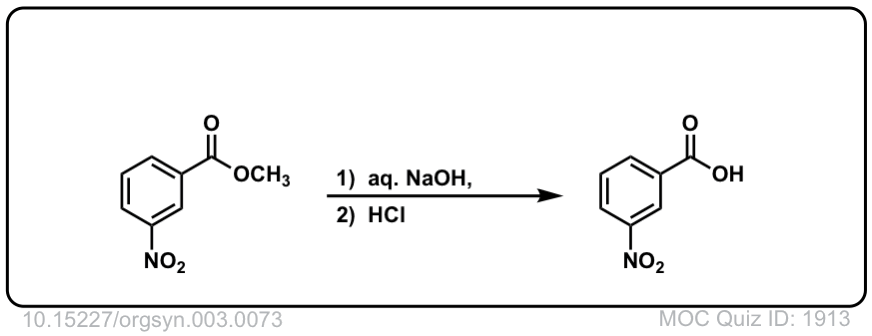
Org. Synth. 1923, 3, 73
DOI Link: 10.15227/orgsyn.003.0073
 Click to Flip
Click to Flip
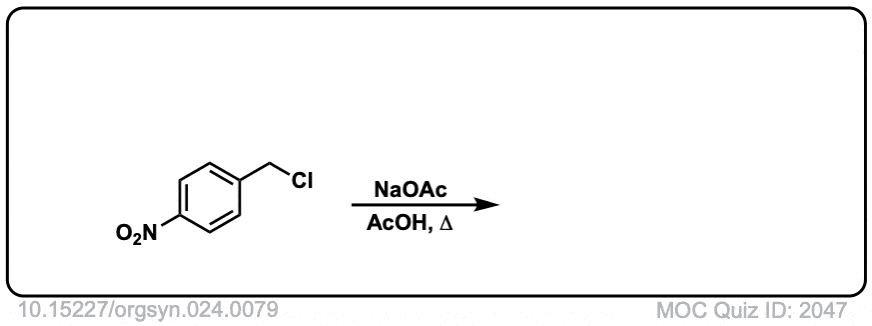
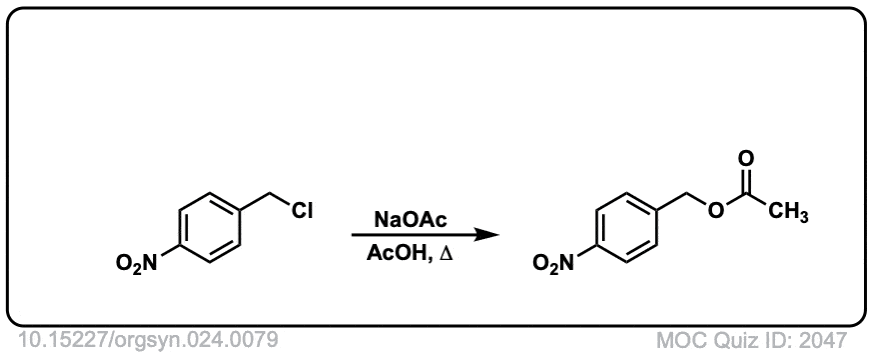
Org. Synth. 1944, 24, 81
DOI Link: 10.15227/orgsyn.024.0081
 Click to Flip
Click to Flip
Org. Synth. 1944, 24, 81
DOI Link: 10.15227/orgsyn.021.0099
 Click to Flip
Click to Flip
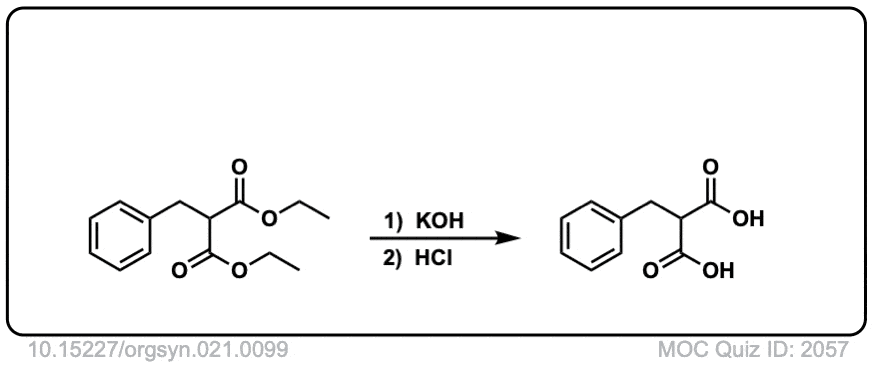
Quiz 2057-OS-III-pg-705-2
Org. Synth. 1958, 38, 47
DOI Link: 10.15227/orgsyn.038.0047
 Click to Flip
Click to Flip
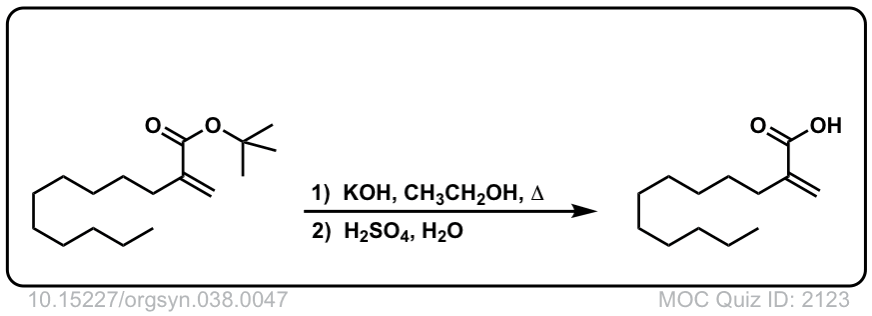
Using another equivalent of base is also fine.
For the mechanism in step 3, does the deprotonation always occur using the alkoxide or can another equivalent of base remove the H on the carboxylic acid and then give this H to the alkoxide? (I know this is a more roundabout way)
I’m a little confused with the third example since it started with 5 carbons and ended with 6 carbons
Fixed, thank you!
Is this really not an equilibrium? Because it says it is in my textbook for mechanism of Saponification of esters
It’s not an equilibrium if the ester is being treated with NaOH, no. Once the carboxylic acid forms, it will be deprotonated to give the carboxylate and it stays there.
Now, if instead of NaOH, an alkoxide is used (like NaOCH3 or NaOEt) THEN the reaction will be in equilibrium between two esters. That’s called, “transesterification” which is slightly different : – )