Bromination of Aromatics to give Bromoarenes
Description: Treatment of an aromatic such as benzene (Br2) and a Lewis acid (such as AlBr3 or FeBr3) leads to formation of the bromobenzene.
The rest of this page is available to MOC Members only.
To get access to this page, plus over 2500 quizzes, the Reaction Encyclopedia, Org 1 / Org 2 summary sheets, and flashcards, sign up here for only 30 cents/ day!
Real-Life Examples:
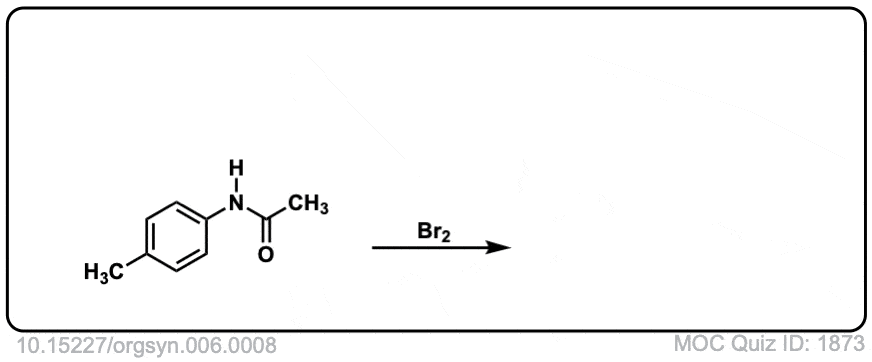
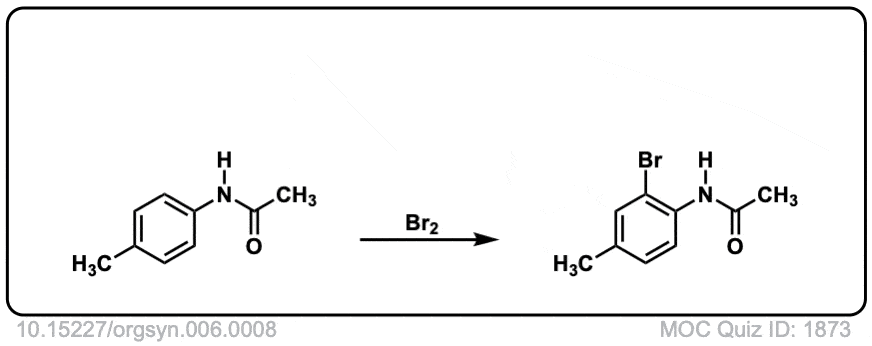
Org. Synth. 1926, 6, 8
DOI Link: 10.15227/orgsyn.006.0008
 Click to Flip
Click to Flip
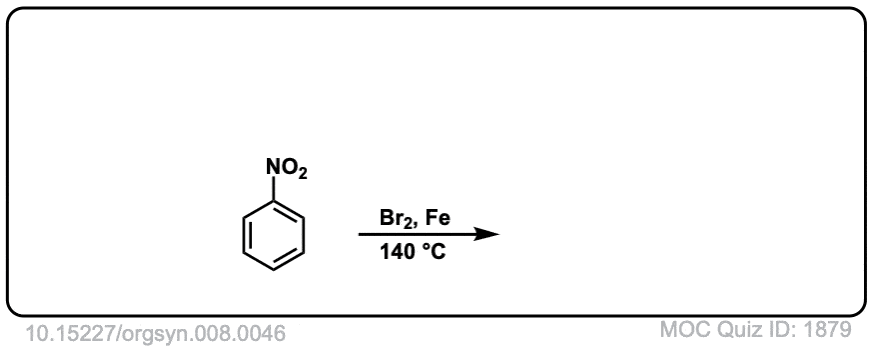
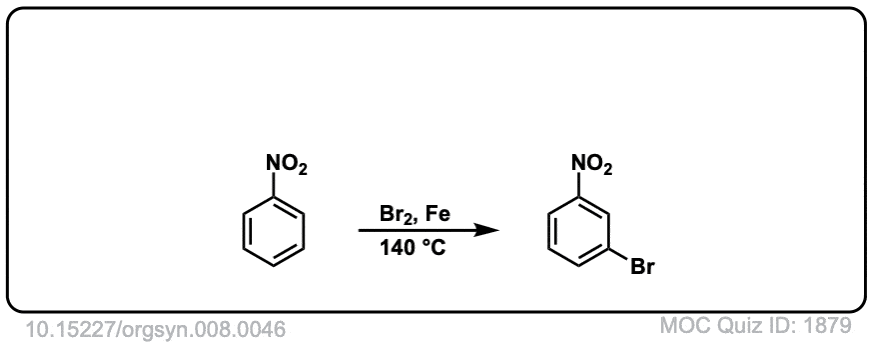
Org. Synth. 1928, 8, 46
DOI Link: 10.15227/orgsyn.008.0046
 Click to Flip
Click to Flip
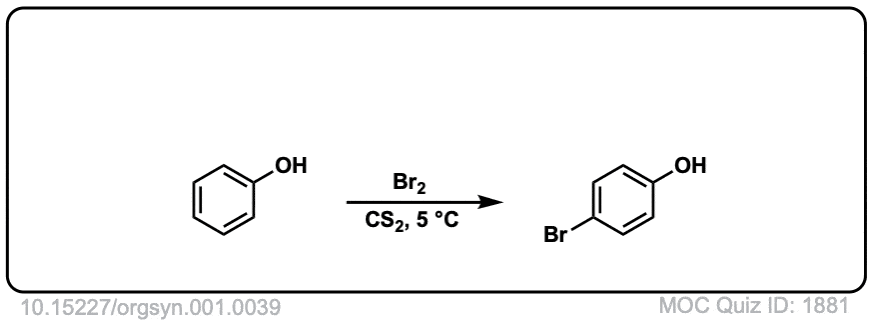
Org. Synth. 1921, 1, 39
DOI Link: 10.15227/orgsyn.001.0039
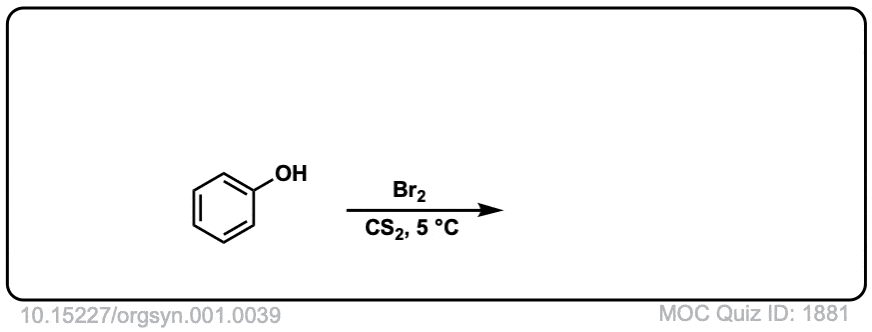 Click to Flip
Click to Flip
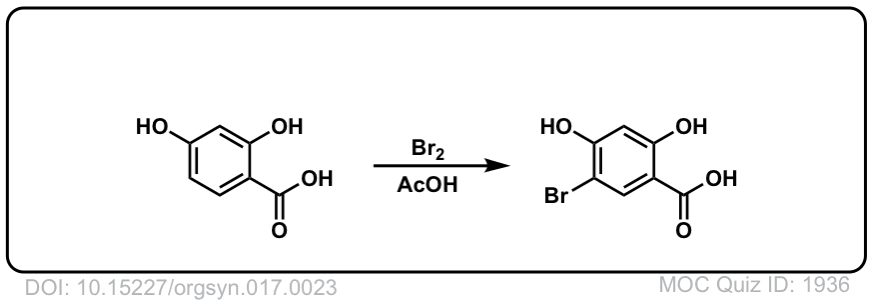
Org. Synth. 1937, 17, 23
DOI Link: 10.15227/orgsyn.017.0023
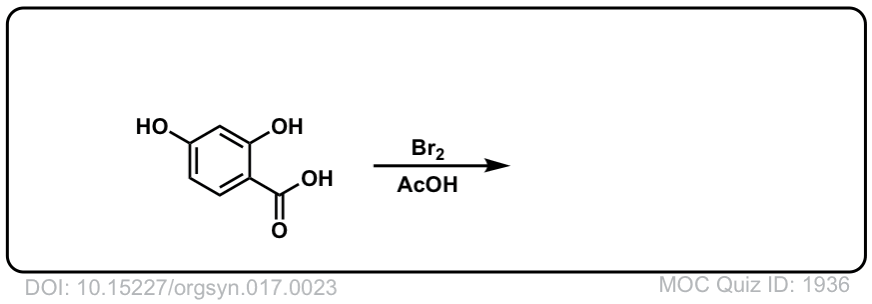 Click to Flip
Click to Flip
Org. Synth. 1951, 31, 29
DOI Link: 10.15227/orgsyn.031.0029
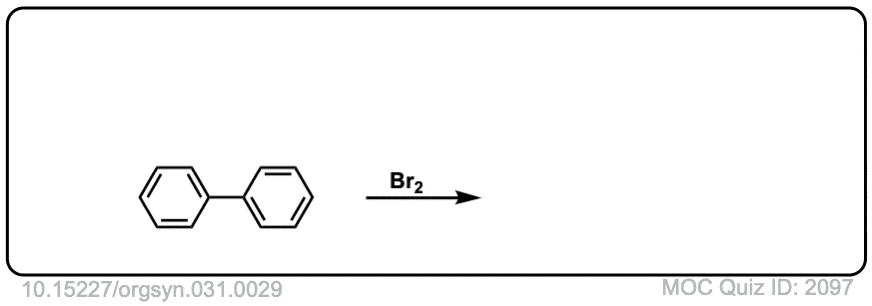 Click to Flip
Click to Flip
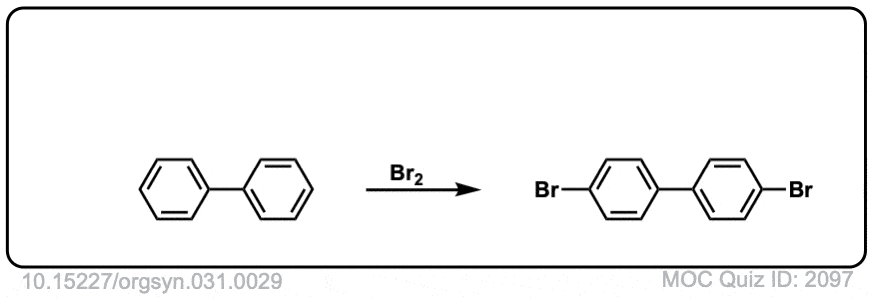
Org. Synth. 1960, 40, 7
DOI Link: 10.15227/orgsyn.040.0007
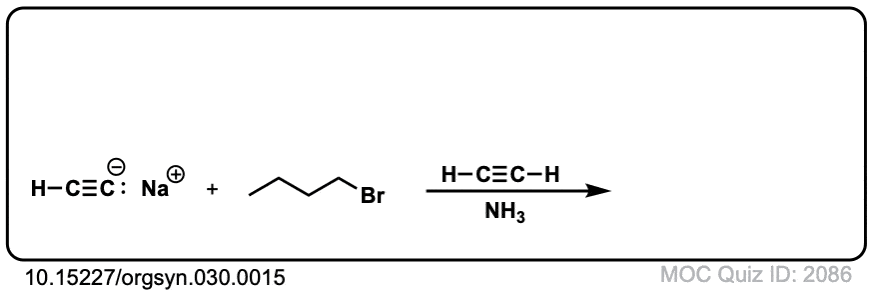 Click to Flip
Click to Flip
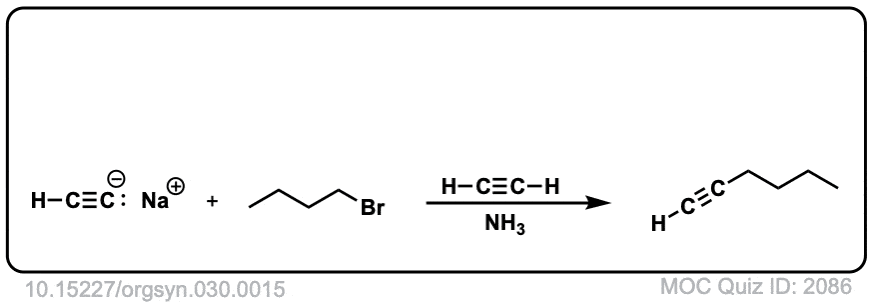
I think picture four has two products
The bromination of nitrobenzene? This should only give one product, the meta- substituted product.
First picture should include the reaction of bromination, not chlorination
Fixed – thank you very much