Clemmensen Reduction of Ketones/Aldehydes to Alkanes
Description: Addition of zinc amalgam, Zn(Hg) and acid to a ketone results in an alkane. This is called the Clemmensen reduction.
The rest of this page is available to MOC Members only.
To get access to this page, plus over 2500 quizzes, the Reaction Encyclopedia, Org 1 / Org 2 summary sheets, and flashcards, sign up here for only 30 cents/ day!
Real-World Example:
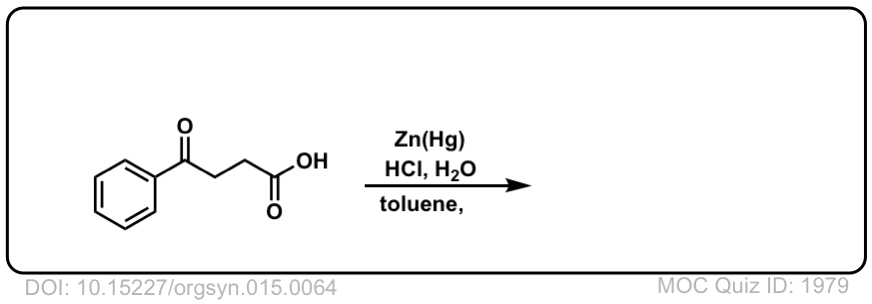
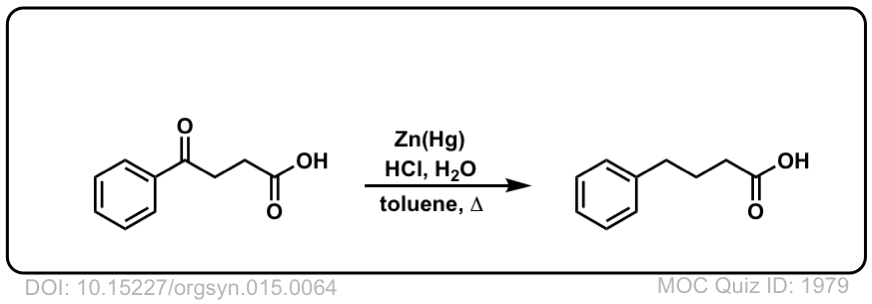
Org. Synth. 1935, 15, 64
DOI Link: 10.15227/orgsyn.015.0064
 Click to Flip
Click to Flip
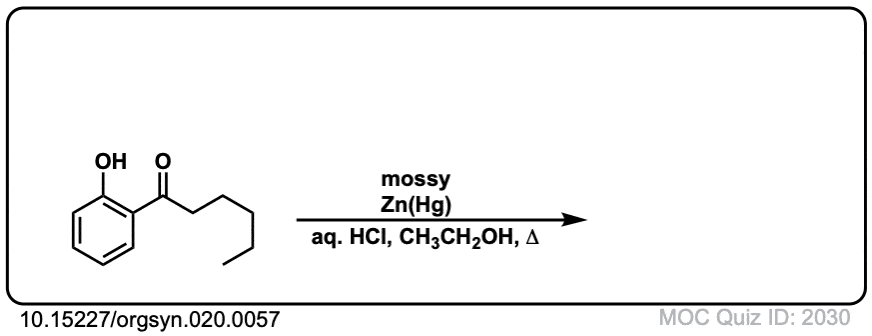
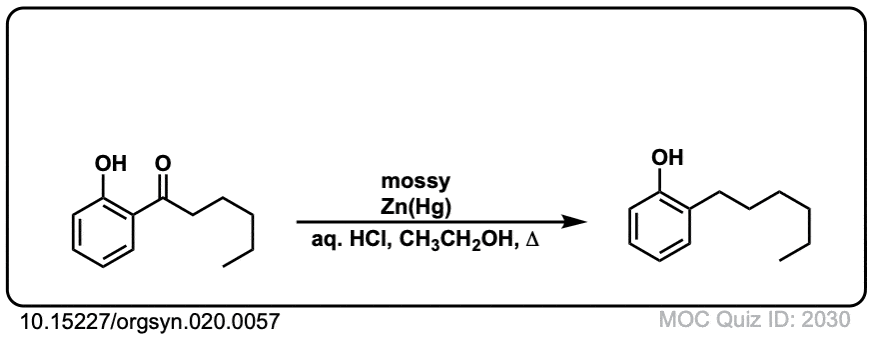
Org. Synth. 1940, 20, 57
DOI Link: 10.15227/orgsyn.020.0057
 Click to Flip
Click to Flip
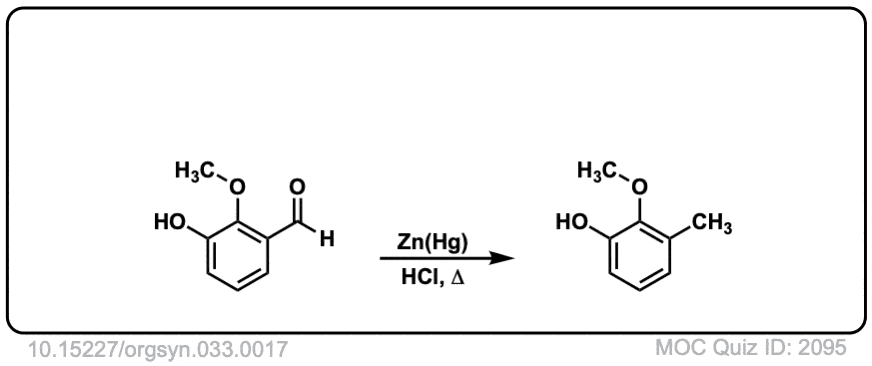
Org. Synth. 1953, 33, 17
DOI Link: 10.15227/orgsyn.033.0017
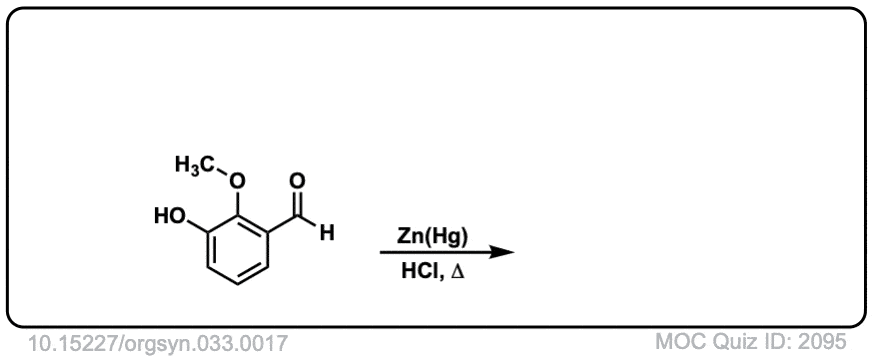 Click to Flip
Click to Flip
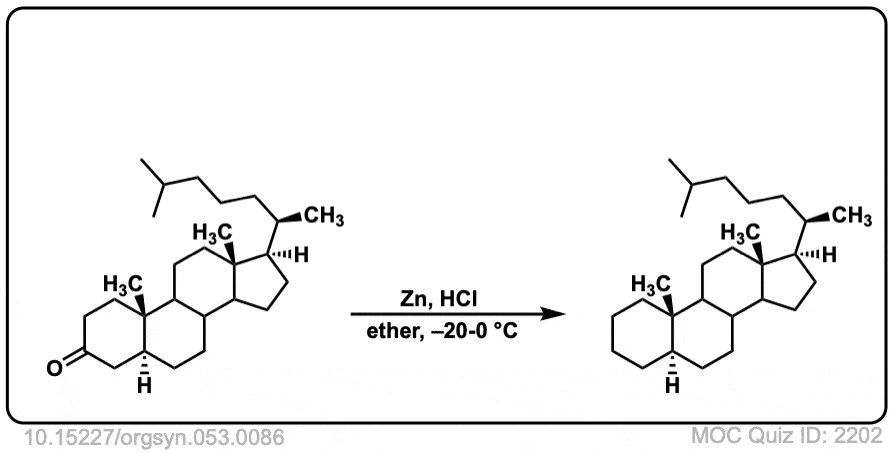
Org. Synth. 1973, 53, 86
DOI Link: 10.15227/orgsyn.053.0086
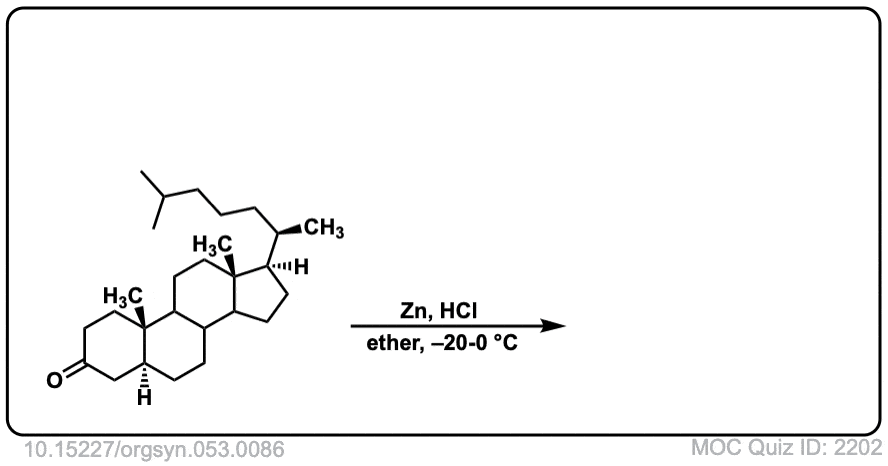 Click to Flip
Click to Flip
Hello, wouldn’t the cyclic acetal in the fourth example be hydrolyzed in acidic conditions?
That’s a good question. In the lab, that’s certainly something to be concerned about. There’s a lot of room for interpretation as to what “H3O+” means. If it’s brief treatment with aqueous acid, then no, the acetal won’t hydrolyze. If it’s extended treatment at high temperature with very concentrated aqueous acid, then yes, the acetal will hydrolyze.
There’s certainly an argument to be made that the acetal will hydrolyze, but this is an exam-type exercise (rather than a real life example) and instructors will vary on their opinions on this.
(The book Protective Groups in Organic Synthesis by Peter Wuts has details on things like half-lives for various acetal groups in the presence of various acidic conditions that help bench chemists to make actual decisions about these things).
I honestly have no idea. I’m still in high school so I have no idea about the Dowd-Beckwith expansion reaction, however the Wikipedia page did throw some light on it. However I think that shouldn’t be happening, since we aren’t using a alkyl halide side chain or AlBN.
This page seems to offer an explanation, stating 1,3 dicarbonyls form ring contraction products via cyclopropanediols, trapped as acetates: http://bit.ly/1CI3nhj
I still haven’t fully understood it though.
My org chem professor told me that clemmensen reduction of 1,3 dicarbonyl compounds gives a differently rearranged compound. Like 3,3-dimethyl-1,5-cyclohexanedione, gives 1,3,3-trimethyl-cyclopentanone (less membered ring). And 2,4-pentanedione gives 2-methyl-3-butanone. Could you explain to me the mechanism of these reactions and suggest ways to write the product for any such dicarbonyl clemmensen reduction?
That is seriously funky. I wonder if it’s generating a ketyl radical and then going through a cyclopropane, much like some versions of the Dowd-Beckwith rearrangement? http://en.wikipedia.org/wiki/Dowd–Beckwith_ring-expansion_reaction
I honestly have no idea. I’m still in high school so I have no idea about the Dowd-Beckwith expansion reaction, however the Wikipedia page did throw some light on it. However I think that shouldn’t be happening, since we aren’t using a alkyl halide side chain or AlBN.
This page seems to offer an explanation, stating 1,3 dicarbonyls form ring contraction products via cyclopropanediols, trapped as acetates: http://bit.ly/1CI3nhj
I still haven’t fully understood it though.
Also, in my previous comment I seem to have gotten the IUPAC names wrong. It should have been 5,5-dimethyl-1,3-cyclohexanedione, 2,4,4-trimethyl cyclopentanone and 3-methyl-2-butanone.
On an Orgo 2 HW question we had to propose the synthesis of 1-bromo-3-propylbenzene. This was a multiple choice question, so we had to figure out the order of using the clemmensen reduction, bromination of arenes, and friedel crafts. What kept throwing me off was what order to put them in. It’s tricky! Could you explain how to approach that?
Thanks so much for your help, this is a great site and is extremely useful!