Conversion of carboxylic acids into acid chlorides with SOCl2
Description: Thionyl chloride converts carboxylic acids into acid chlorides. The reaction liberates HCl and SO2 gas.
The rest of this page is available to MOC Members only.
To get access to this page, plus over 2500 quizzes, the Reaction Encyclopedia, Org 1 / Org 2 summary sheets, and flashcards, sign up here for only 30 cents/ day!
Real-World Examples:
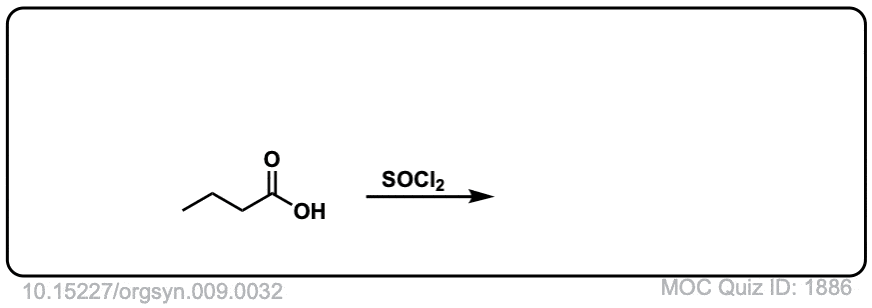
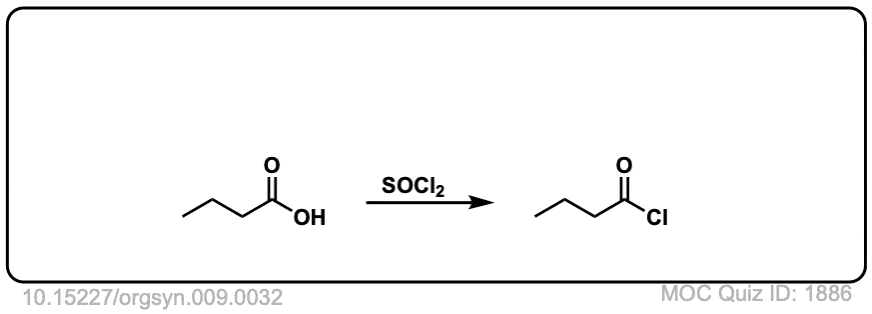
Org. Synth. 1929, 9, 32
DOI Link: 10.15227/orgsyn.009.0032
 Click to Flip
Click to Flip
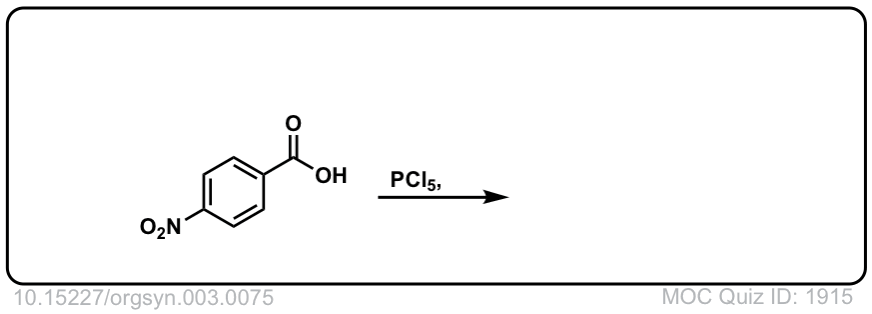
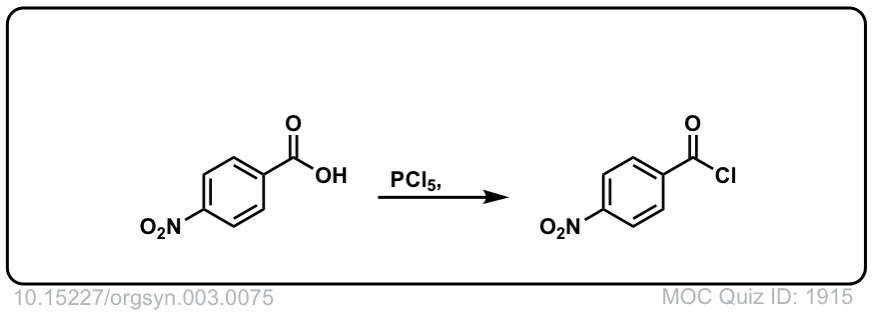
Org. Synth. 1923, 3, 75
DOI Link: 10.15227/orgsyn.003.0075
 Click to Flip
Click to Flip
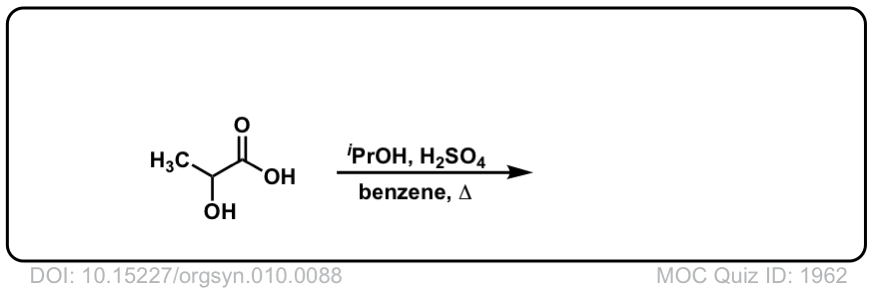
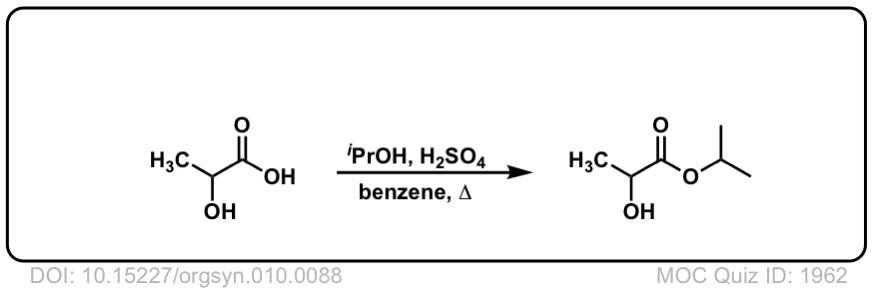
Org. Synth. 1930, 10, 88
DOI Link: 10.15227/orgsyn.010.0088
 Click to Flip
Click to Flip
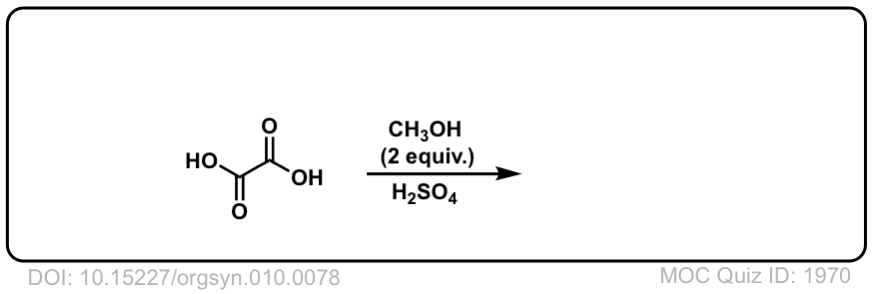
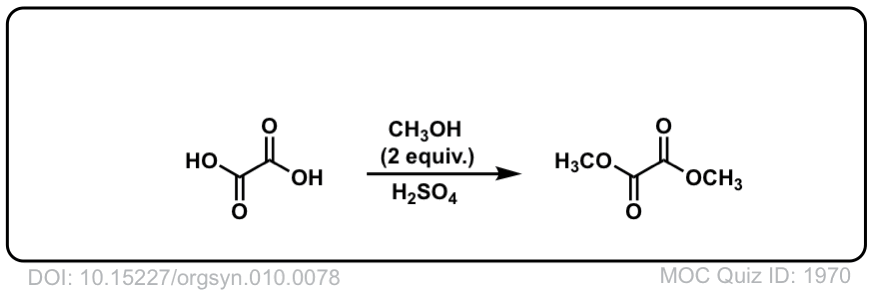
Org. Synth. 1930, 10, 78
DOI Link: 10.15227/orgsyn.010.0078
 Click to Flip
Click to Flip
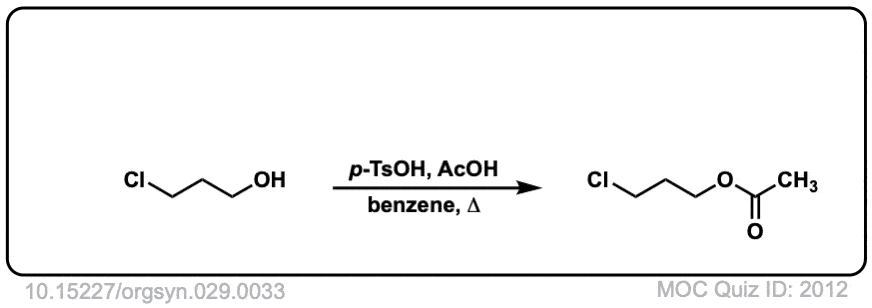
Org. Synth. 1949, 29, 33
DOI Link: 10.15227/orgsyn.029.0033
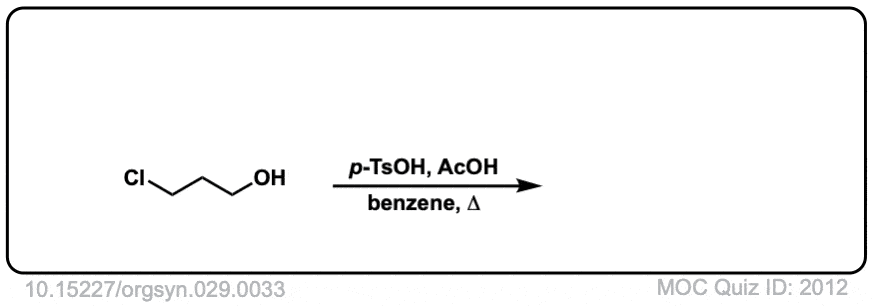 Click to Flip
Click to Flip
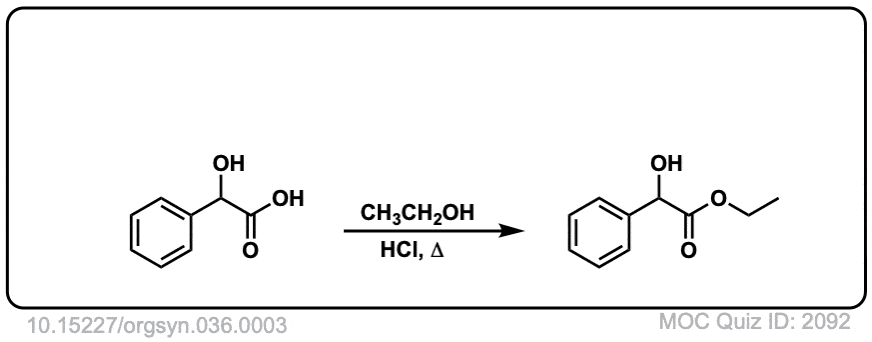
Org. Synth. 1956, 36, 3
DOI Link: 10.15227/orgsyn.036.0003
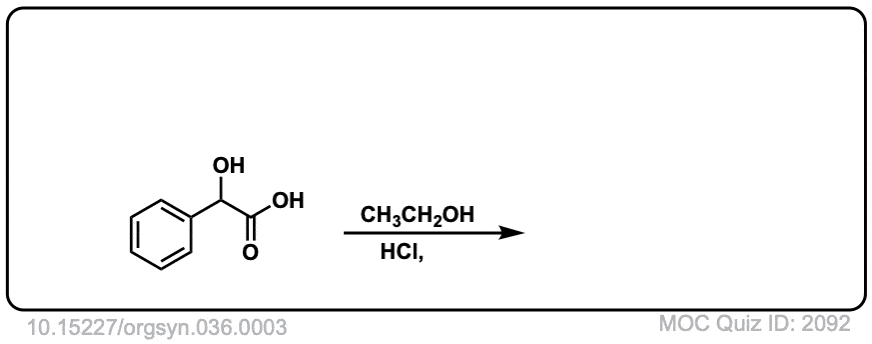 Click to Flip
Click to Flip
can you not use PBr3 to do this aswell?
Yes – you could use PBr3 to make an acid bromide.
What kind of solution should this reaction be done in?
It’s often done in thionyl chloride as solvent. See the Org Syn reference. http://www.orgsyn.org/demo.aspx?prep=CV1P0147
(note that these days it’s more common to use oxalyl chloride w/ DMF as a catalyst under Vilsmeier conditions).