Cope Rearrangement
Description: The Cope rearrangement is a [3,3]-sigmatropic rearrangement of 1,5-dienes. Heating the 1,5-diene results in a reversible rearrangement reaction.
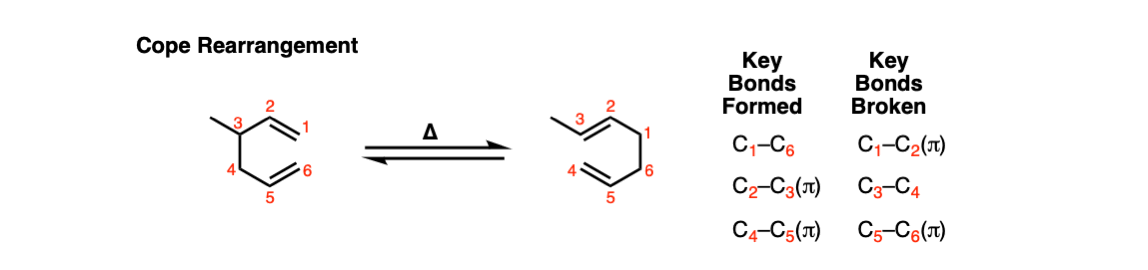
Notes: Not shown above, but if there is an OH on the 3-position, the resulting product is an enol, which then tautomerizes into a carbonyl. This is known as the Oxy-Cope rearrangement and is generally not reversible.
Examples:
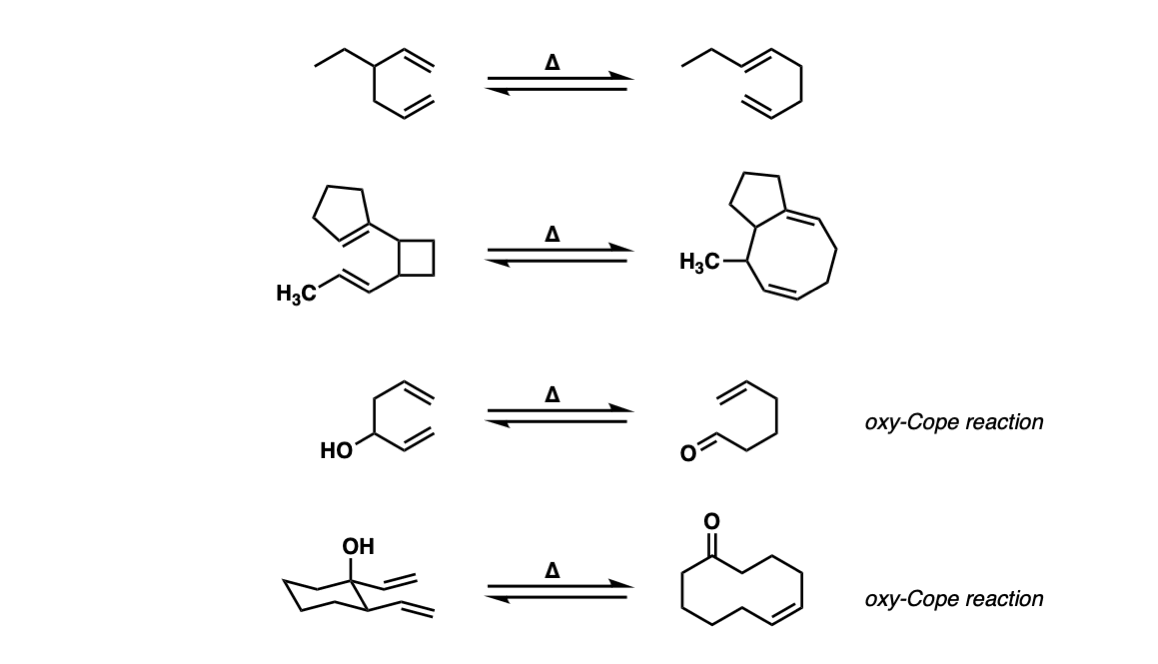
Notes: Example 1 is a fairly typical Cope rearrangement. The product will be favored slightly since it is a more substituted alkene (recall that alkene stability increases as the number of attached carbons increases).
The second example involves the opening of a strained ring (cyclobutane) which has a strong driving force due to the release of ring strain in cyclobutane (ca. 26 kcal/mol).
Example 4 is an Oxy-cope reaction, which forms an enol that then tautomerizes to an aldehyde. The last example shows an Oxy-Cope where a new 10-membered ring is formed.
Mechanism:
Like all pericyclic reactions, the Cope rearrangement occurs in a single step with a concerted transition state.
In this step (Step 1, arrows A, B, and C) two C-C pi bonds are broken along with a single C-C sigma bond, and two C-C pi bonds are formed along with a single C-C sigma bond.
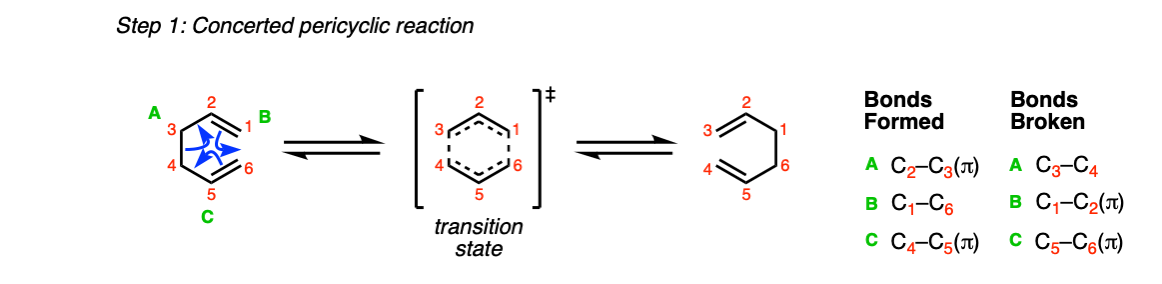
Here’s the Oxy-Cope in action:
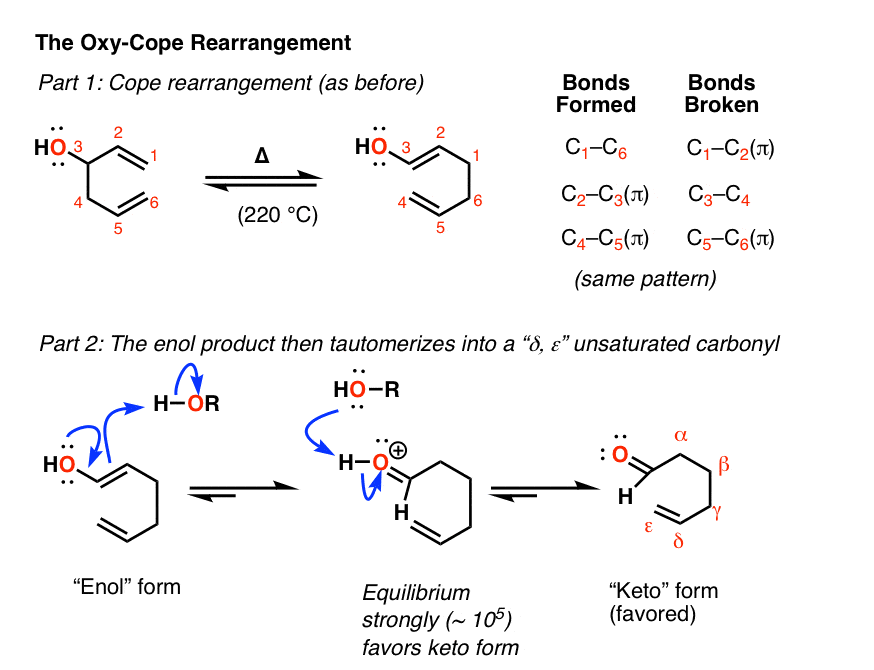
Notes: In most cases, heat is generally required to get the Cope rearrangement to occur since it has a fairly high activation energy (about 33 kcal/mol). A typical value is about 150°C.
For more, see this post: The Cope and Claisen Rearrangements