Dehydration of amides to give nitriles
Description: Primary amides can be converted to nitriles with a dehydrating reagent such as P2O5 .
The rest of this page is available to MOC Members only.
To get access to this page, plus over 2500 quizzes, the Reaction Encyclopedia, Org 1 / Org 2 summary sheets, and flashcards, sign up here for only 30 cents/ day!
Real-Life Examples:
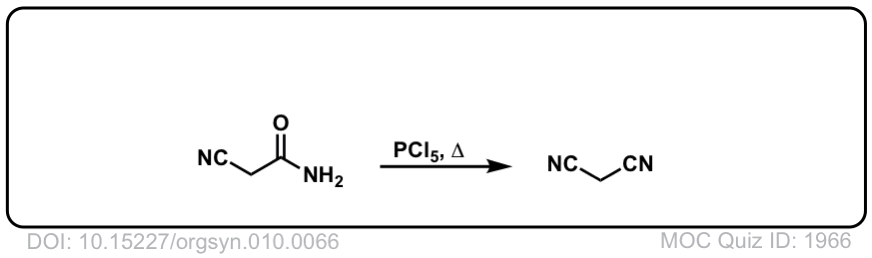
Org. Synth. 1930, 10, 66
DOI Link: 10.15227/orgsyn.010.0066
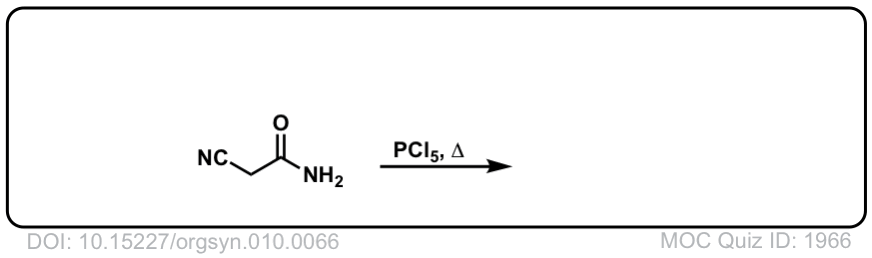 Click to Flip
Click to Flip
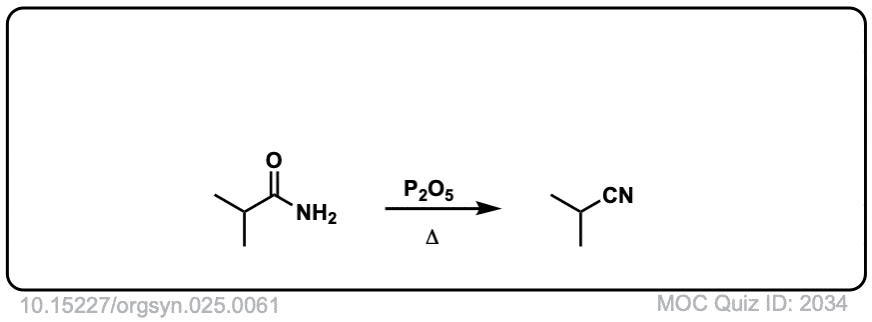
Org. Synth. 1945, 25, 61
DOI Link: 10.15227/orgsyn.025.0061
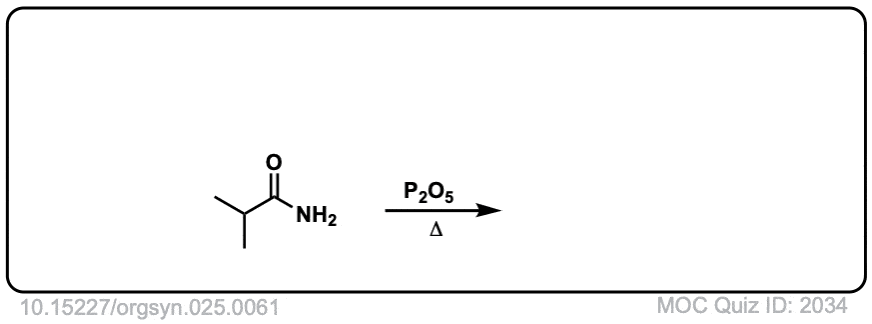 Click to Flip
Click to Flip
Org. Synth. 1945, 25, 63
DOI Link: 10.15227/orgsyn.025.0063
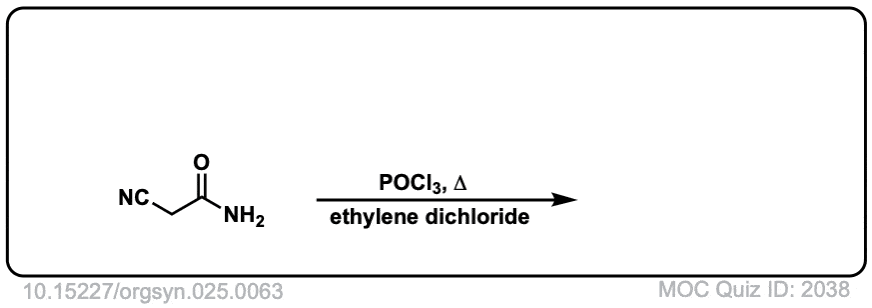 Click to Flip
Click to Flip
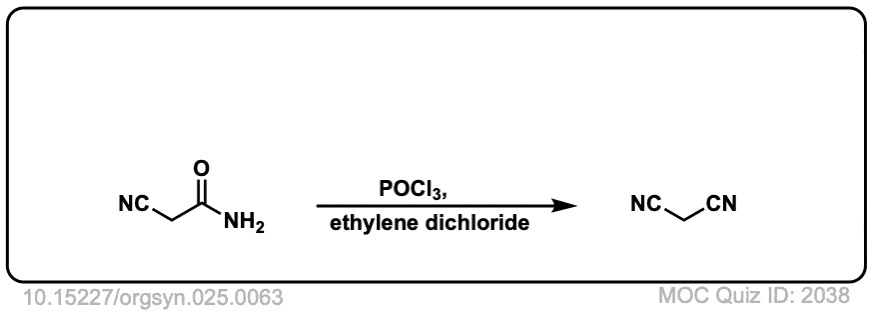
Org. Synth. 1950, 30, 22
DOI Link: 10.15227/orgsyn.030.0022
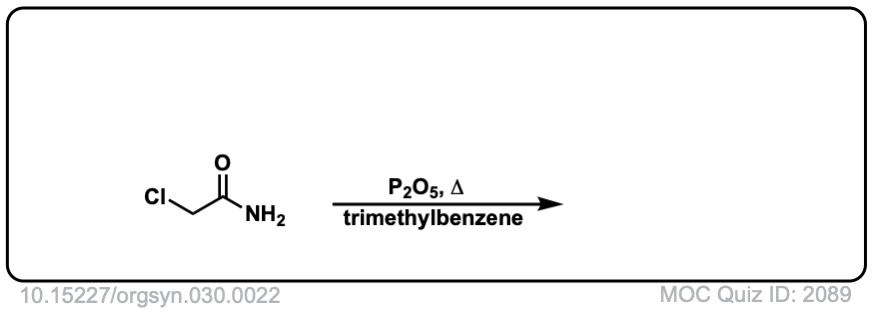 Click to Flip
Click to Flip
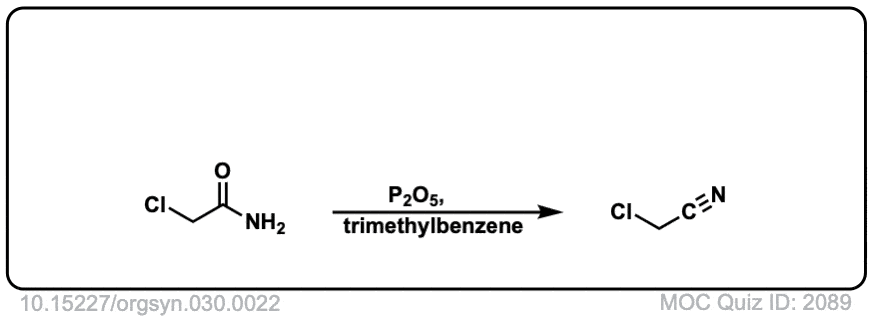
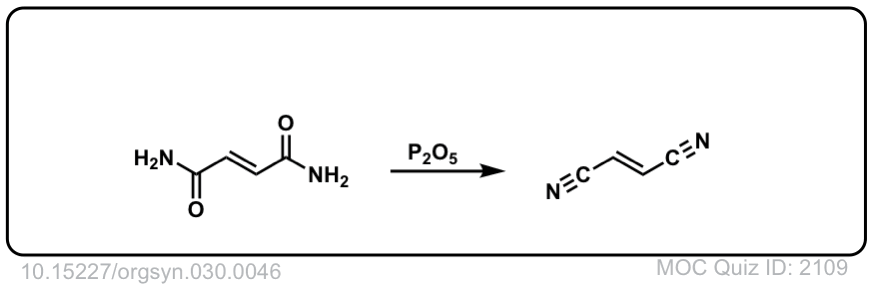
Org. Synth. 1950, 30, 46
DOI Link: 10.15227/orgsyn.030.0046
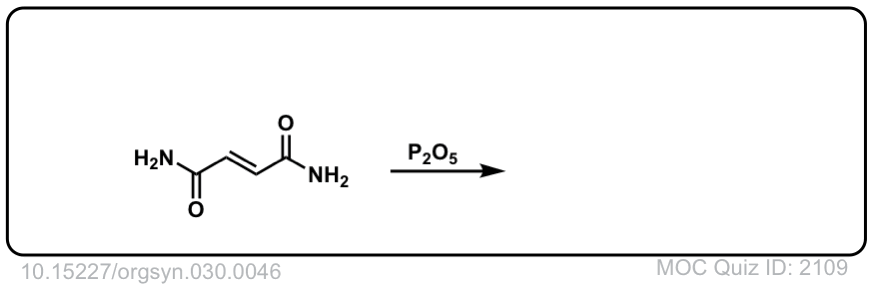 Click to Flip
Click to Flip
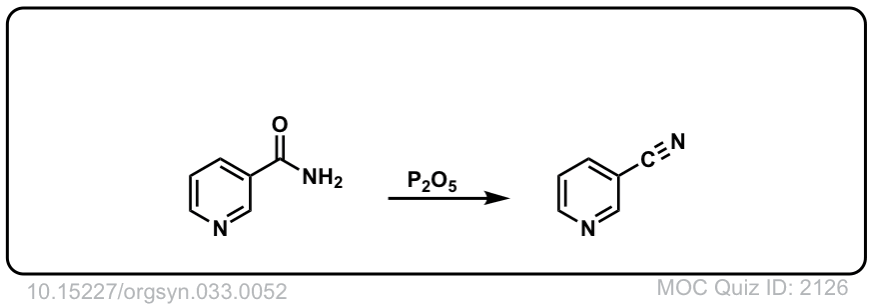
Org. Synth. 1953, 33, 52
DOI Link: 10.15227/orgsyn.033.0052
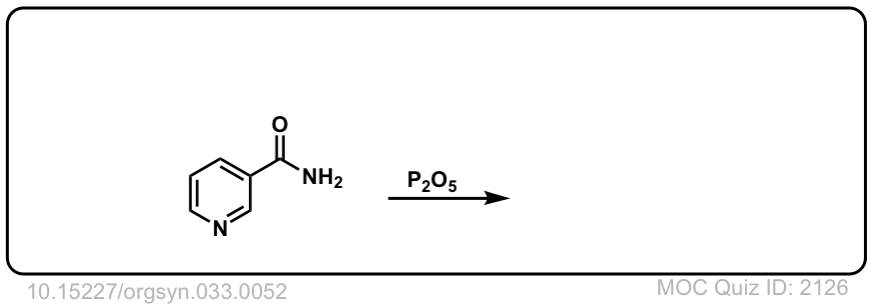 Click to Flip
Click to Flip
how to convert the 2-amino melonamide to 2-amino melononitrile, can we use P2O5? or is there any specific reagent for the above particular reaction?
You would definitely want to protect the amine first.
What is the role of oxygen in the reagent?
Phosphate ions are great leaving groups. Oxygen stabilizes the negative charge.
In step 3, why lone pair on nitrogen attack the carbon ( arrow F) ?
You can look at this mechanism as “elimination”, or, “1,2-elimination”. It’s kicking off a good leaving group (the phosphate) and forming a new C-N pi bond in the process.
Hi James,
My question may sound silly,but i seriously have no idea.Why ‘P’ in P2O5 is attracted by the pi bonds and not the lone pairs of Amine group?
Lone pair of amide is strongly donating into carbonyl carbon – there is pseudo double bond character between carbon and nitrogen. Oxygen is very nucleophilic.