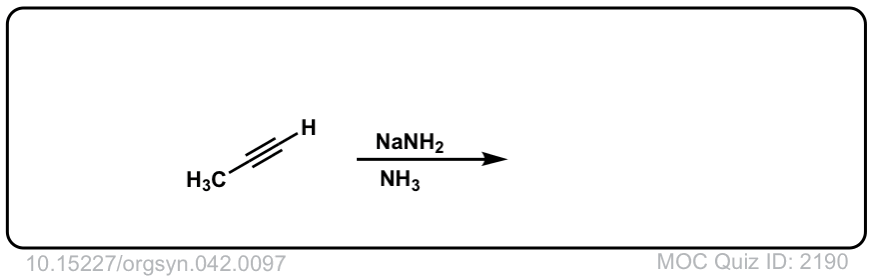
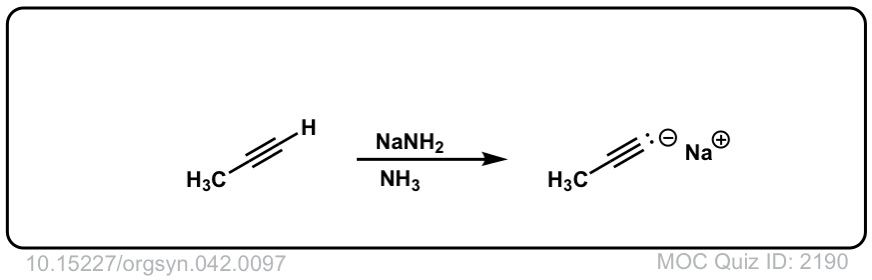
Deprotonation of alkynes with base to give acetylide ions
Description: Treatment of an acetylene with a strong base such as NaNH2 results in acetylide ions
The rest of this page is available to MOC Members only.
To get access to this page, plus over 2500 quizzes, the Reaction Encyclopedia, Org 1 / Org 2 summary sheets, and flashcards, sign up here for only 30 cents/ day!
Real-Life Example:
Org. Synth. 1962, 42, 97
DOI Link: 10.15227/orgsyn.042.0097
 Click to Flip
Click to Flip
Is there really no reaction for example 4? what about the zipper reaction to move the non-terminal triple bond to terminal?
Zipper reaction generally uses potassium alkoxides. Also, have never seen it covered in introductory undergraduate organic chemistry.
No problem!
Also in this: “Note that NH3 is the byproduct here. NaNH2 or KNH3…” it should be KNH2.
Thank you!
In the second example, the cation in the product should be K+.
Thank you
Why wouldnt a base such as tBuCl work in this case to deprotonate a terminal alkyne?
tBuCl is not a base; do you mean t-BuO(-) ? Given the large pKa difference between tBuoH (17) and alkyne (25) the equilibrium will lie on the side of t-BuO- , so it’s more useful to use a strong base like NaNH2 (pka of conjugate acid 38) to deprotonate the alkyne irreversibly.
I’ve got an exam question problem: So I’ve added n-BuLi to an alkyne, from this a suspect I product lithium acetylide, however the question asks for ANOTHER reagent in the reaction, could it be LiH4-, to get rid of the lithium or is there something im missing?