Elimination of water from alcohols to form alkenes using acid
Description: Addition of acid to alcohols can result in elimination to form the corresponding alkene along with a molecule of water.
The rest of this page is available to MOC Members only.
To get access to this page, plus over 2500 quizzes, the Reaction Encyclopedia, Org 1 / Org 2 summary sheets, and flashcards, sign up here for only 30 cents/ day!
Real-Life Examples:
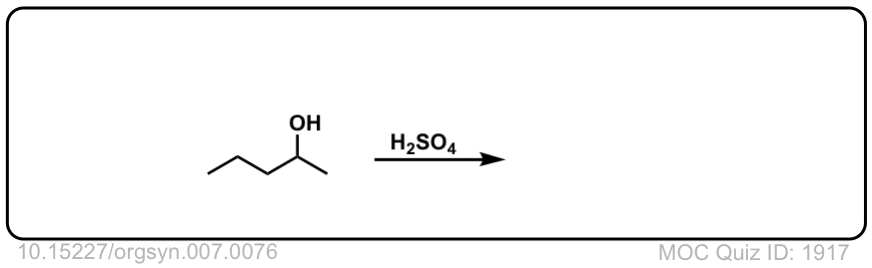
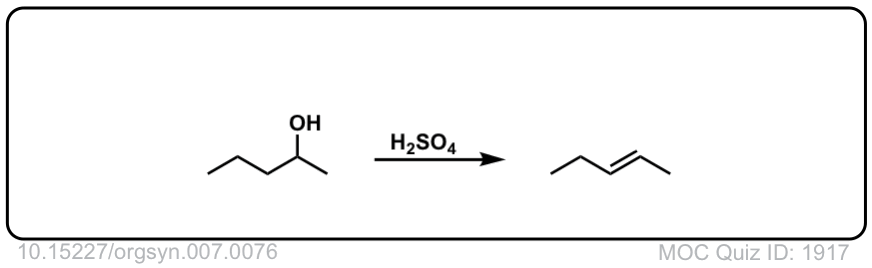
Org. Synth. 1927, 7, 76
DOI Link: 10.15227/orgsyn.007.0076
 Click to Flip
Click to Flip
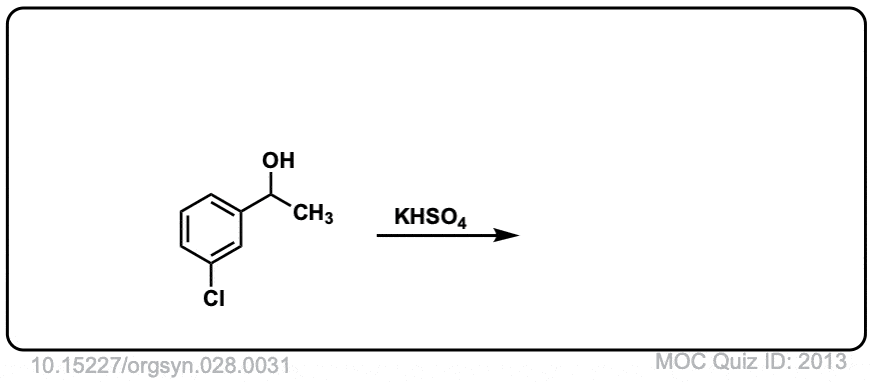
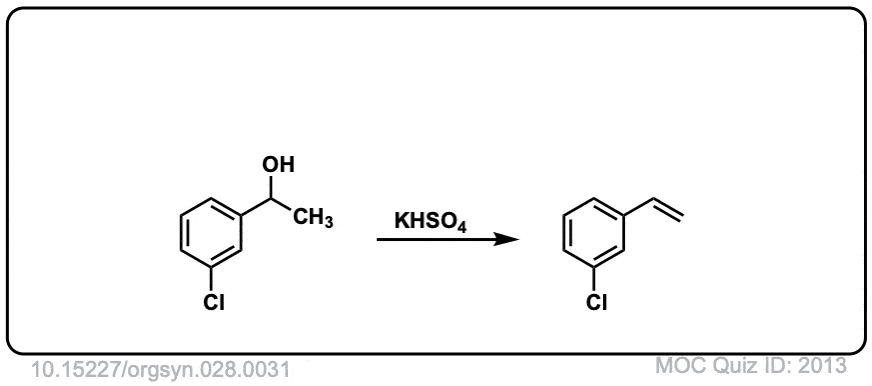
Org. Synth. 1948, 28, 31
DOI Link: 10.15227/orgsyn.028.0031
 Click to Flip
Click to Flip
thank you !!
Hello
From what I get, here, Sulfuric acid and phosphoric acid play the same role. Is there a point in usng both for a synthesis of an alkene from an alcohol ? Thank you
This website is a life-saver
Yes, they can be considered equivalent for our purposes.
James, in the note above you state “this works best for secondary and tertiary Alkyl Halides”… I know you mean alcohols, yes?
Yes! Thanks, I’ll fix this.
When would you want to use this method of alcohol elimination INSTEAD of POCl3?
Only in extremely simple cases, because of the chances for rearrangements.