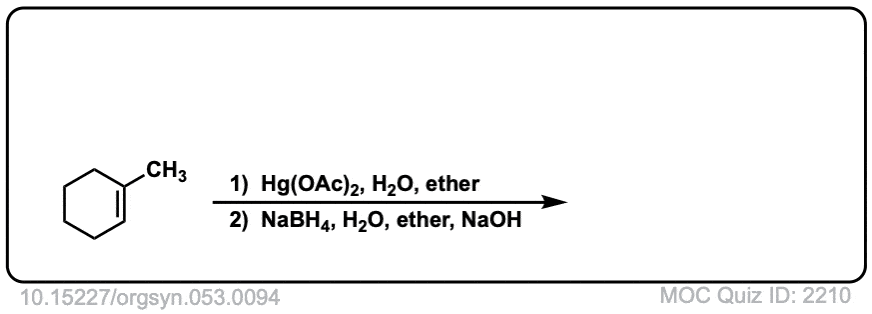
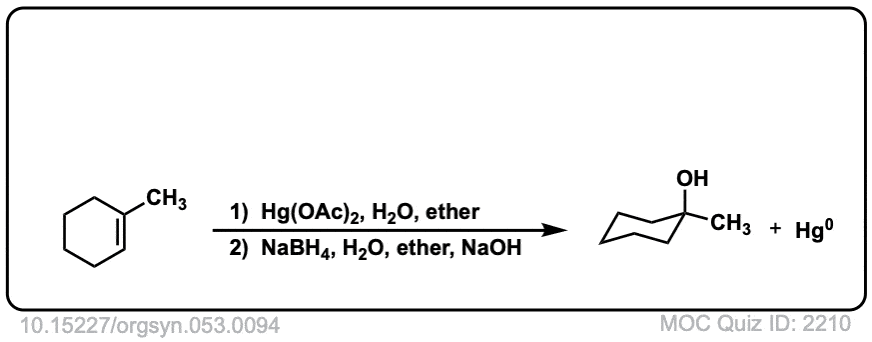
Oxymercuration: Alcohols from alkenes using Hg(OAc)2 and Water
Description: Alkenes treated with mercuric acetate [ Hg(OAc)2 ] and water will be hydrated to form alcohols, after addition of NaBH4. The rest of this page is available to MOC Members only.
To get access to this page, plus over 2500 quizzes, the Reaction Encyclopedia, Org 1 / Org 2 summary sheets, and flashcards, sign up here for only 30 cents/ day!
Real-Life Example:
Org. Synth. 1973, 53, 94
DOI Link: 10.15227/orgsyn.053.0094
 Click to Flip
Click to Flip
Hi James,
“Removal of mercury leads to the formation of a free radical (Step 6) so the reaction is not stereoselective.”
Could you explain this a lottle more? How could this result in different chirality at the carbon that will bond to hydrogen?
Say you have a stereogenic carbon center (aka “chiral carbon”) containing a C-H bond with the (R) configuration. Let’s subject that C-H bond to homolytic cleavage, forming a new radical.
What happens to the stereochemistry at that carbon? Does it remain (R) ?
When this has been done, experiments show that the configuration at the carbon is not retained. Instead, a mixture of inversion and retention of configuration is observed (there are rare exceptions, such as at the bridgehead carbon of bicyclic molecules)
The proposed reason is that free radicals have a shallow trigonal pyramidal structure, which inverts rapidly at room temperature (like an umbrella in a stiff wind). This means that the radical can react on either face of the carbon (BTW, it can be simpler to just imagine the free radical as being “flat”, like a carbocation).
So in the removal of the C-Hg bond, a new free radical on carbon is formed. This free radical can react with H at either face of the carbon, resulting in a mixture of retention and inversion of configuration at that carbon.
I hope that answers your question?
Can you show how the NaBH_4 reacts with the Hg(OAc)? I am confused on the arrow pushing there.
Hi, you can think of NaBH4 as a source of H(-) and AcO(-) is a good leaving group.
It’s similar to a nucleophilic substitution except on Hg instead of carbon.
So draw an arrow from the lone pair on H(-) towards the Hg. Then (OAc) can be a leaving group. This gives you R-Hg-H
Doesn’t the mercury atom have an extra electron after step 5 (i.e. isn’t it a free radical)?
Why is this homolytic bond cleavage favored?
The mercury-carbon bond is extremely weak (about 30 kcal/mol versus 100 kcal/mol for a C-H bond). Even mild heat will result in homolytic cleavage.
After cleavage of the C-Hg bond, Hg is in the (I) oxidation state. After the radical abstracts the hydrogen, mercury goes to the Hg (0) oxidation state.
Sorry, but can I know why sometimes THF is used in this reaction? Is it purely a polar solvent which can dissolve the organic compound and Mercuric Acetate? Or it has got to do with the stabilising ability of the oxygen atom on the Hg atom? (Something similiar to why Grignard reactions are performed in ether and THF)
Thanks
Kudos dude. Needed this earlier in the course but sleep is overrated anyway.
it is very helpful for student.thanks to you
Nice. Thanks for the radical mech in reduction step, though should it be Hg(l) instead of Hg(s)?
I point out to my students that the environmental impact of Hg(OAc)2 must be balanced against improved yield and avoidance of C+ rearrangements.
Ha!. Yes, I guess it should be Hg(liquid). The times I’ve done it, it formed this beautiful little grey dispersion, it must have stuck in my mind as a solid & I forgot it’s actually a liquid.