Formation of alkynes through double elimination of vicinal dibromides
Description: Sodium amide will convert 1,2-dihalides (“vicinal dihalides”) into alkynes through two consecutive elimination reactions.
The rest of this page is available to MOC Members only.
To get access to this page, plus over 2500 quizzes, the Reaction Encyclopedia, Org 1 / Org 2 summary sheets, and flashcards, sign up here for only 30 cents/ day!
Real-Life Examples:
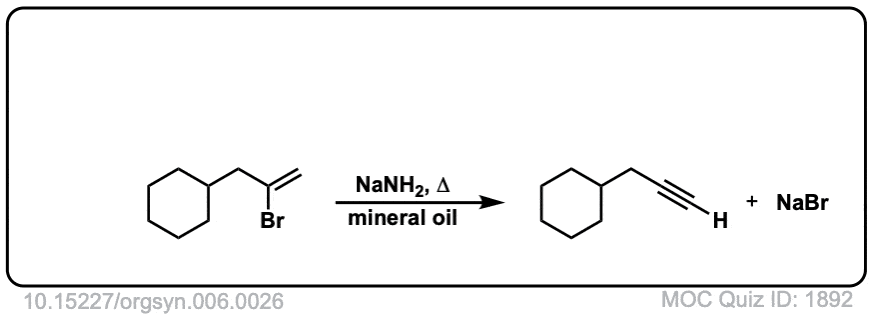
Org. Synth. 1926, 6, 26
DOI Link:10.15227/orgsyn.006.0026
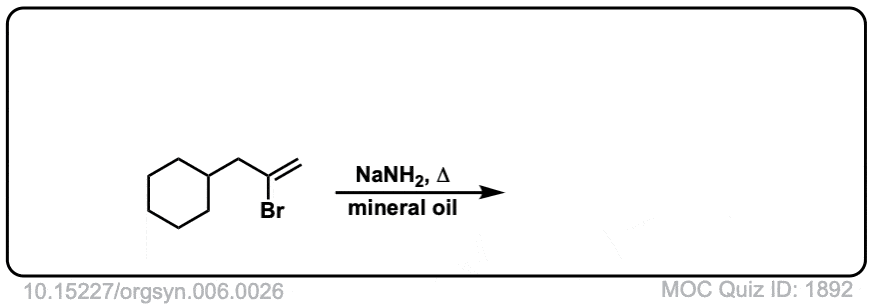 Click to Flip
Click to Flip
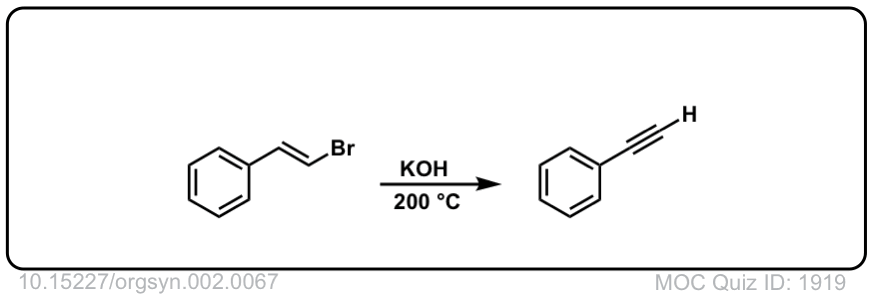
Org. Synth. 1922, 2, 67
DOI Link:10.15227/orgsyn.002.0067
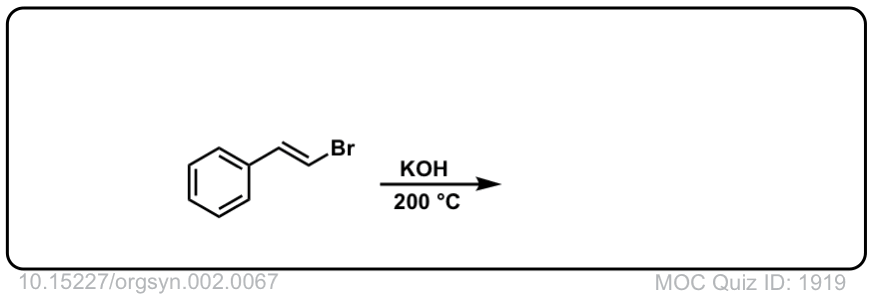 Click to Flip
Click to Flip
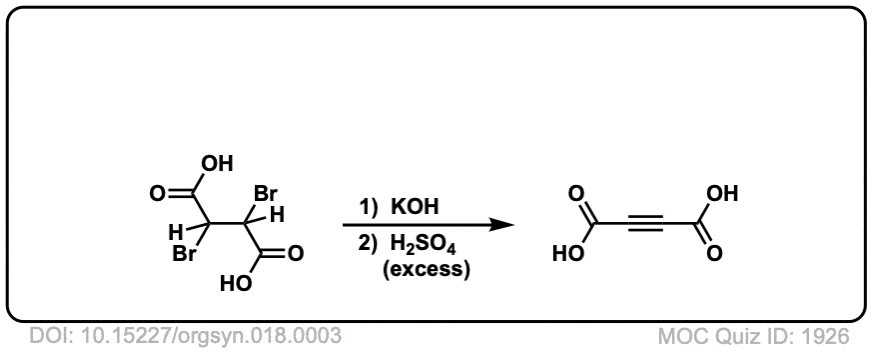
Org. Synth. 1938, 18, 3
DOI Link: 10.15227/orgsyn.018.0003
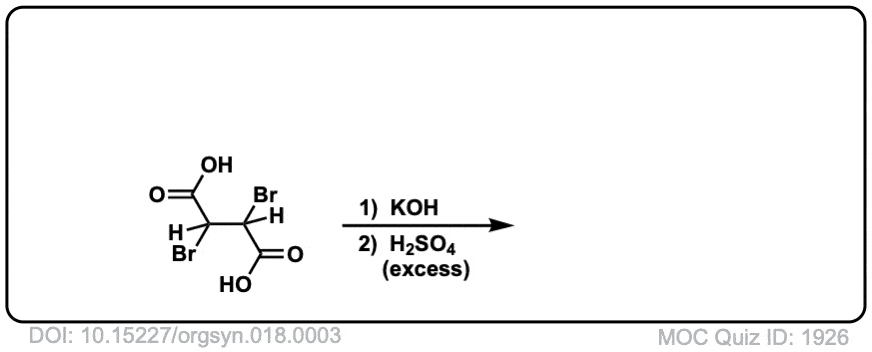 Click to Flip
Click to Flip
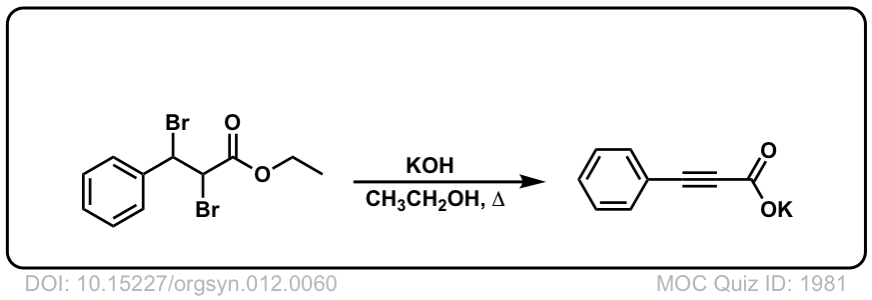
Org. Synth. 1932, 12, 60
DOI Link: 10.15227/orgsyn.012.0060
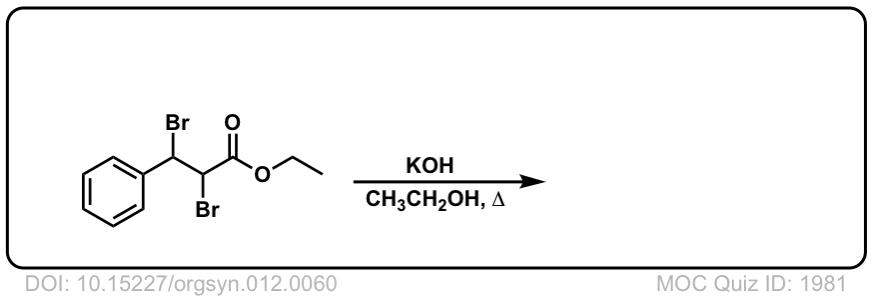 Click to Flip
Click to Flip
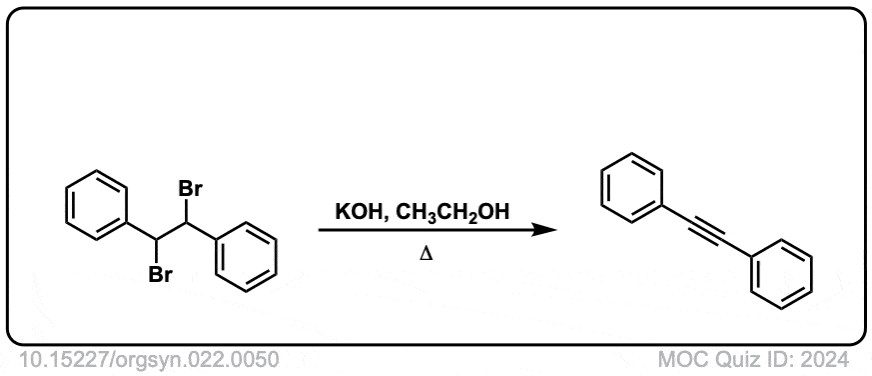
Org. Synth. 1942, 22, 50
DOI Link: 10.15227/orgsyn.022.0050
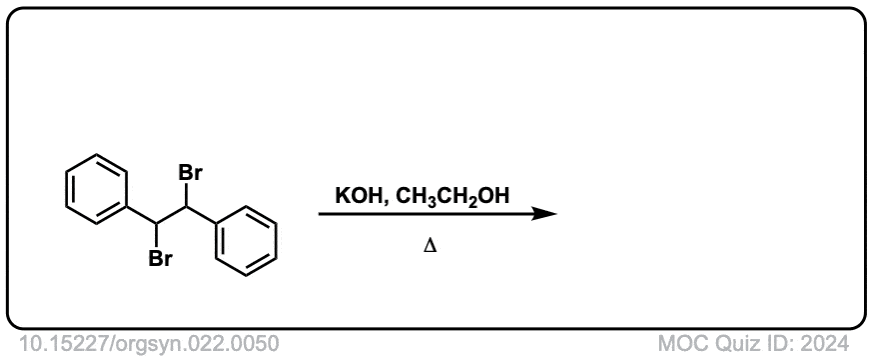 Click to Flip
Click to Flip
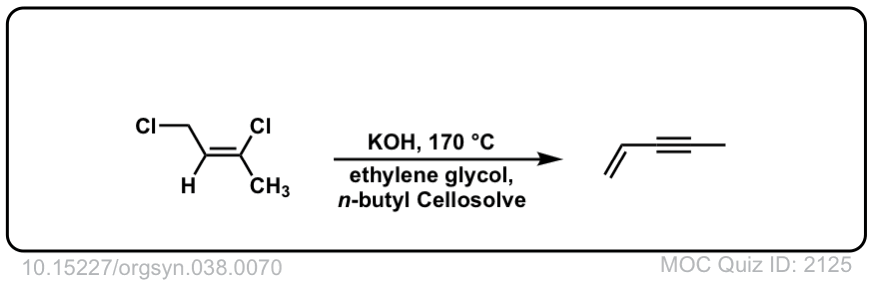
Org. Synth. 1958, 38, 70
DOI Link: 10.15227/orgsyn.038.0070
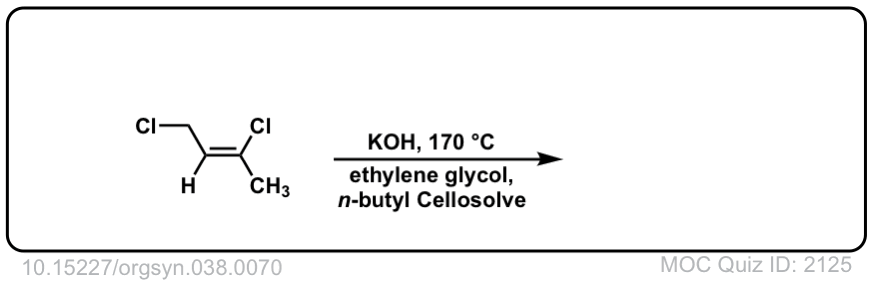 Click to Flip
Click to Flip
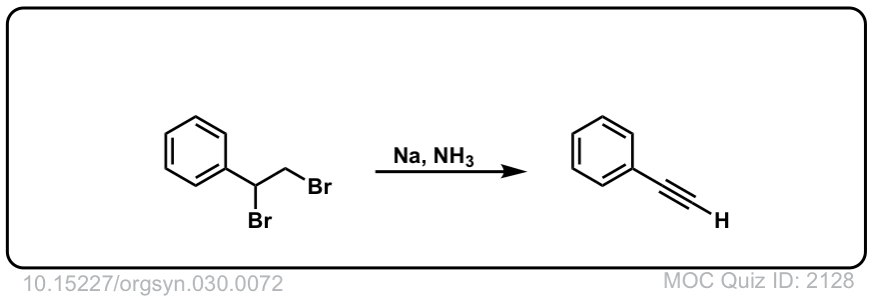
Org. Synth. 1950, 30, 72
DOI Link: 10.15227/orgsyn.030.0072
 Click to Flip
Click to Flip
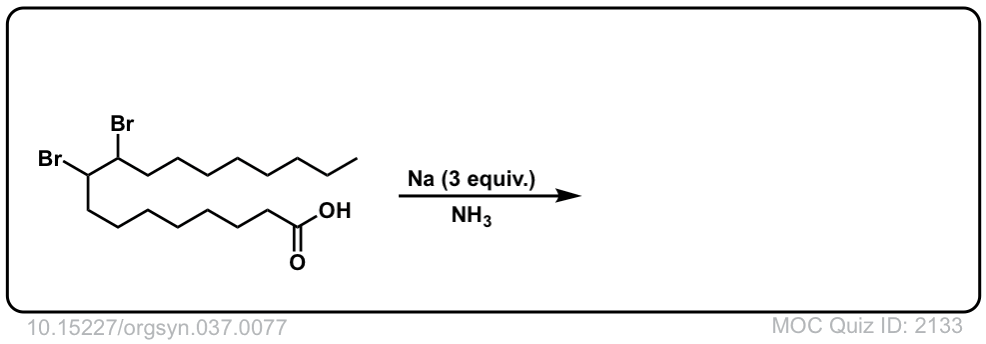
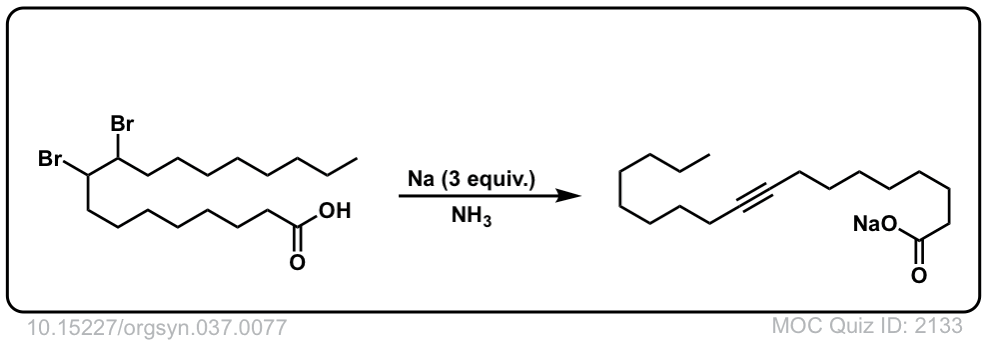
Org. Synth. 1957, 37, 77
DOI Link: 10.15227/orgsyn.037.0077
 Click to Flip
Click to Flip
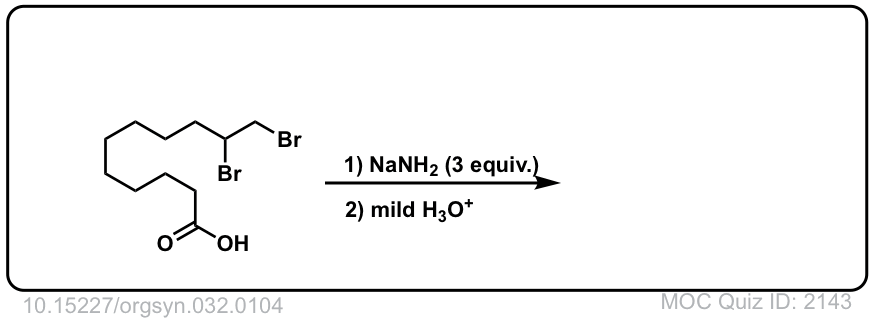
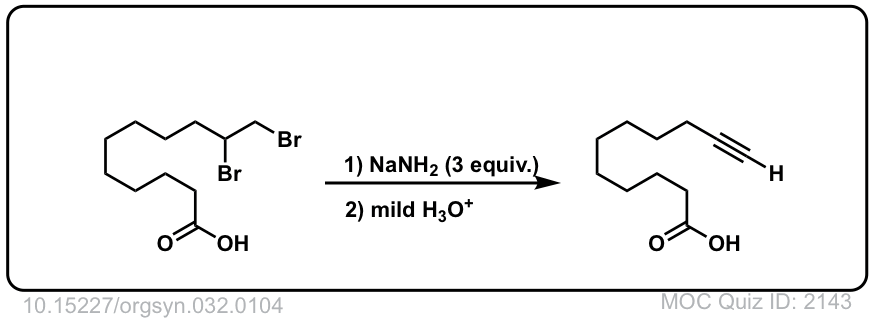
Org. Synth. 1952, 32, 104
DOI Link: 10.15227/orgsyn.032.0104
 Click to Flip
Click to Flip
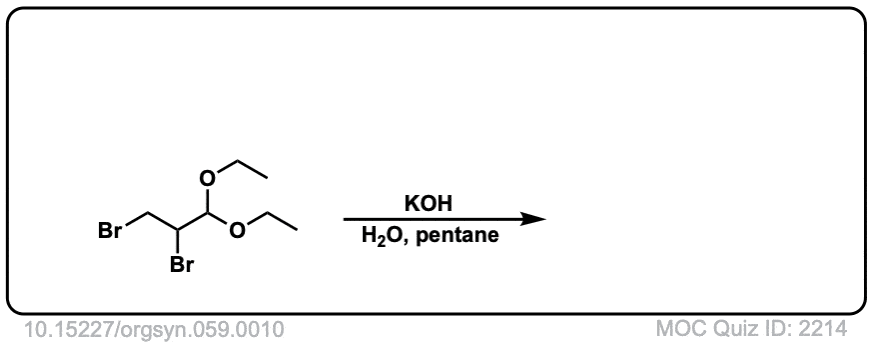
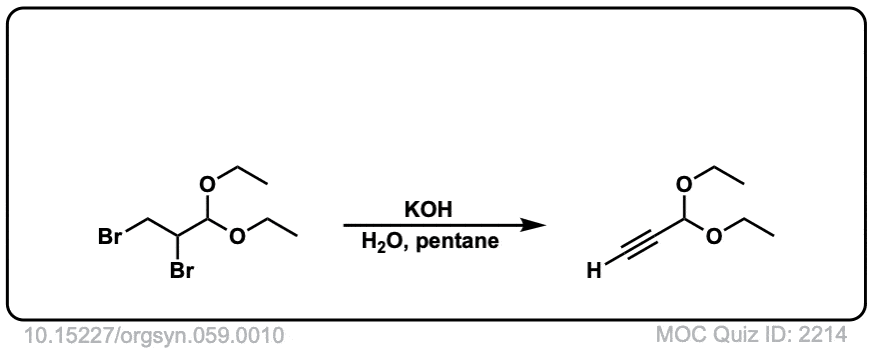
Org. Synth. 1979, 59, 10
DOI Link: 10.15227/orgsyn.059.0010
 Click to Flip
Click to Flip
Does this work the same way for Geminal Dihalides?
Yes it does!
Would this also work with geminal dihalides?
Yes, it should.
My course taught us that we need three molar equivalents of NaNH2 for this reaction, as it prevents the formation of the alkynyl salt.
True, for terminal alkynes. Once the alkyne is formed, it can then be deprotonated by NaNH2 in solution. For practical purposes, three equivalents for the formation of terminal alkynes is best.