Formation of amides from acid chlorides and amines
Description: Addition of primary or secondary amines (or ammonia) to acid chlorides results in amides.
The rest of this page is available to MOC Members only.
To get access to this page, plus over 2500 quizzes, the Reaction Encyclopedia, Org 1 / Org 2 summary sheets, and flashcards, sign up here for only 30 cents/ day!
Real-Life Examples:
Org. Synth. 1929, 9, 16
DOI Link: 10.15227/orgsyn.009.0016
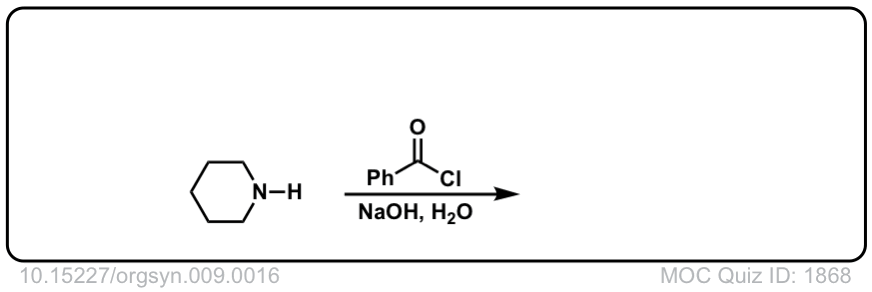 Click to Flip
Click to Flip
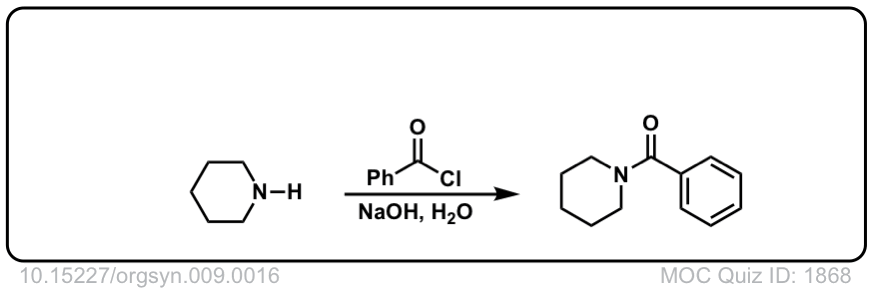
Org. Synth. 1939, 19, 4
DOI Link: 10.15227/orgsyn.019.0004
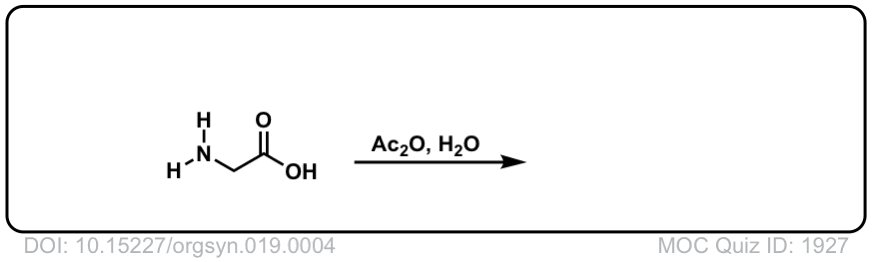 Click to Flip
Click to Flip
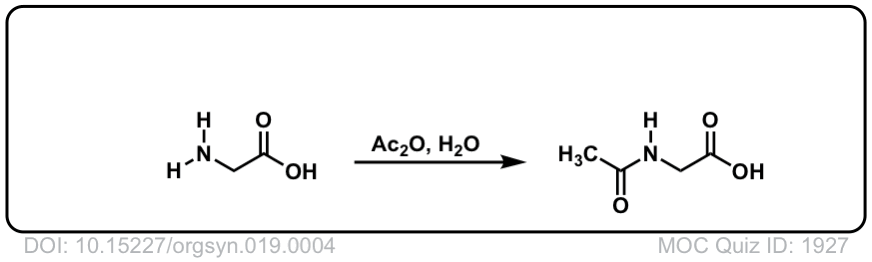
Org. Synth. 1941, 21, 4
DOI Link: 10.15227/orgsyn.021.0004
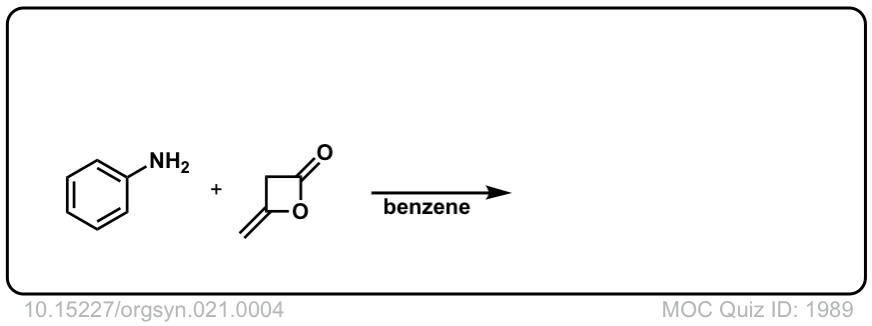 Click to Flip
Click to Flip
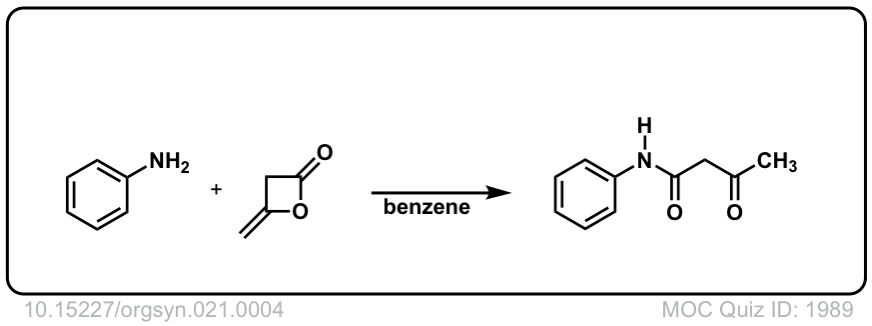
Org. Synth. 1945, 25, 58
DOI Link: 10.15227/orgsyn.025.0058
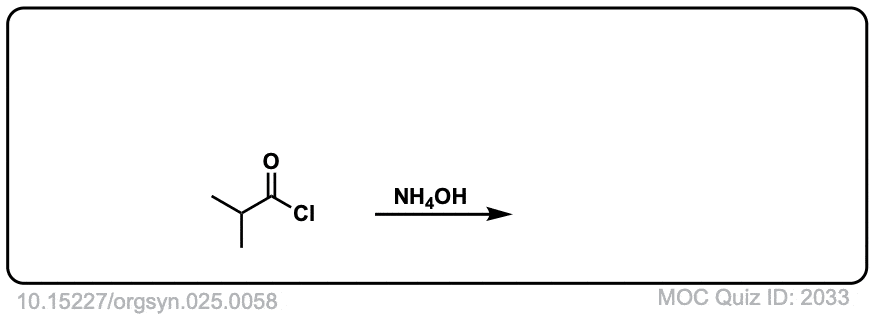 Click to Flip
Click to Flip
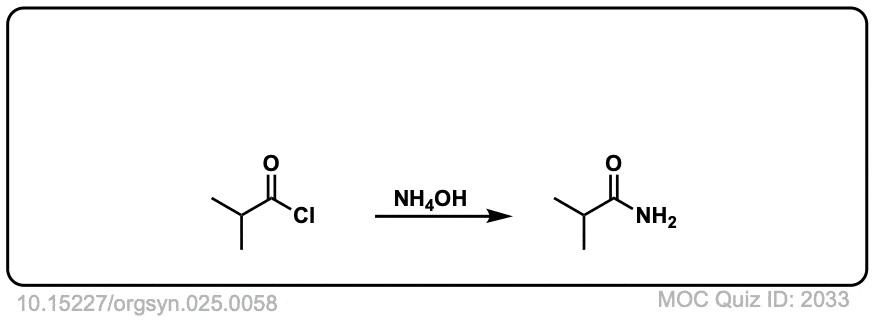
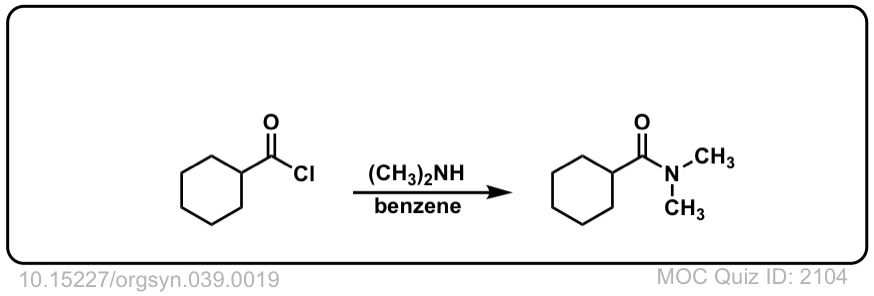
Org. Synth. 1959, 39, 19
DOI Link: 10.15227/orgsyn.039.0019
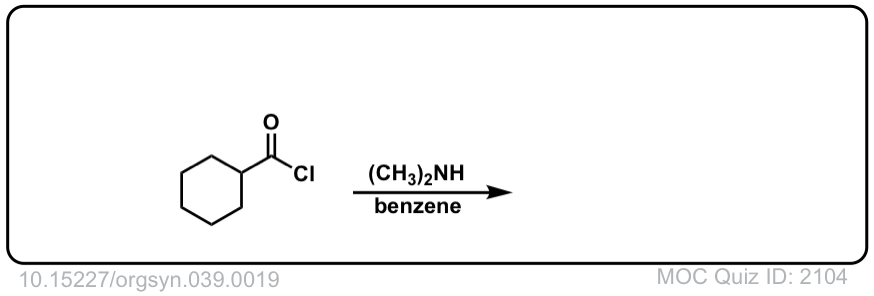 Click to Flip
Click to Flip
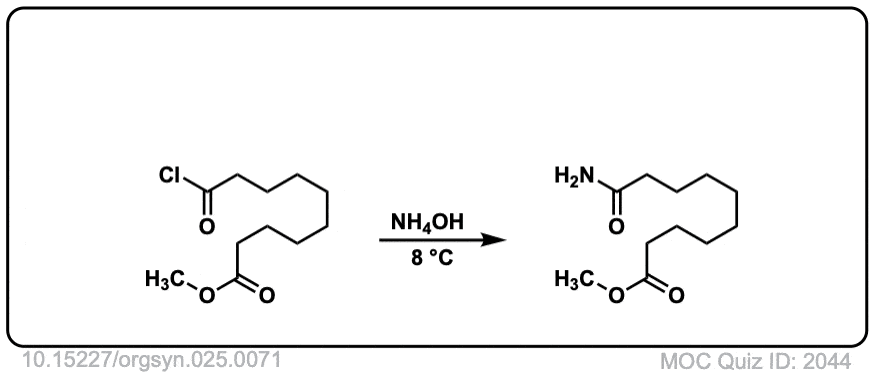
Org. Synth. 1945, 25, 71
DOI Link: 10.15227/orgsyn.025.0071
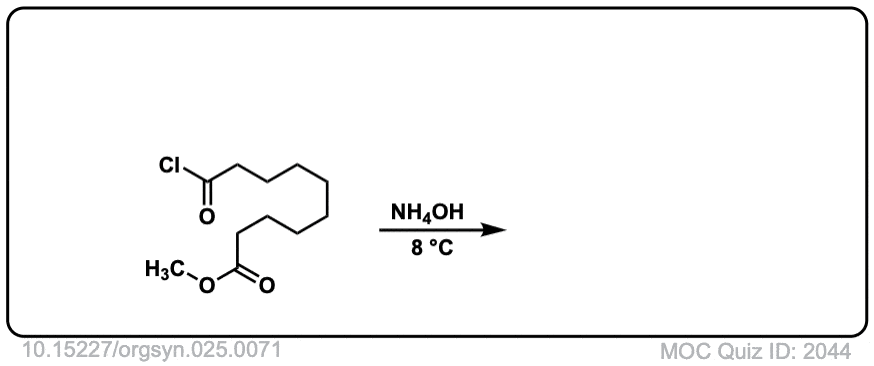 Click to Flip
Click to Flip
Org. Synth. 1945, 25, 78
DOI Link: 10.15227/orgsyn.025.0078
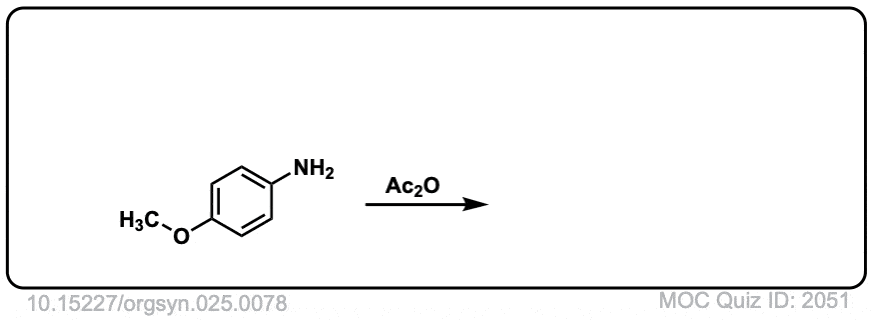 Click to Flip
Click to Flip
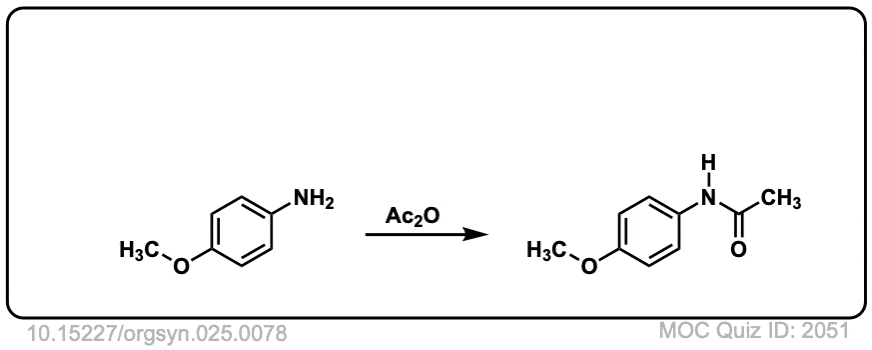
Does this reaction requires a catalyst? and/or special solvent?
No catalyst. Can even be run in biphasic conditions (see: Schotten Baumann reaction) with excess base.
How many moles of amine are required?
At least two, since one of them will react with HCl.
what product would be derived when acid chloride react with triethylamine with tetrahydrofuran as solvent
You’d get an acylammonium salt, which is not going to be very stable.
how can we convert acid halide to peroxide?
To an acyl peroxide? Treat with hydrogen peroxide in the presence of base. The result will be a peroxyacid.
How acid chloride of amino acid synthezed.
For an amino acid like leucine or alanine with non-reactive sidechains, I think you’d need to di-protect the amine as a phthalimide or similar reagent, and then convert it into an acid chloride with SOCl2 or oxalyl chloride. you have to be careful, however – racemization is very facile.