Formation of Bromohydrins from alkenes using water and Br2
Description: Alkenes treated with bromine (Br2) or N-bromosuccinimide (NBS) in the presence of water will form bromohydrins.
The rest of this page is available to MOC Members only.
To get access to this page, plus over 2500 quizzes, the Reaction Encyclopedia, Org 1 / Org 2 summary sheets, and flashcards, sign up here for only 30 cents/ day!
Real-Life Examples:
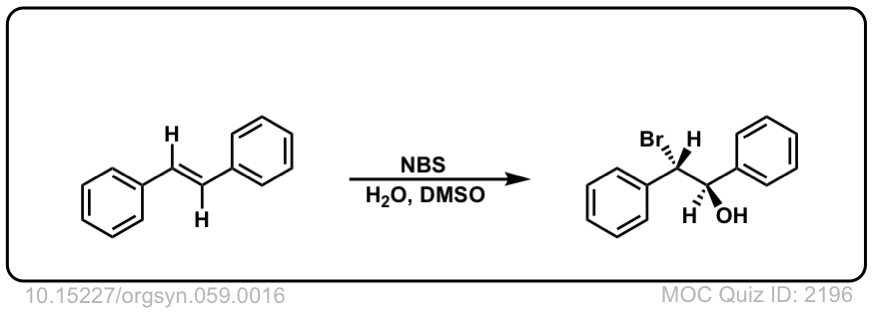
Org. Synth. 1979, 59, 16
DOI Link: 10.15227/orgsyn.059.0016
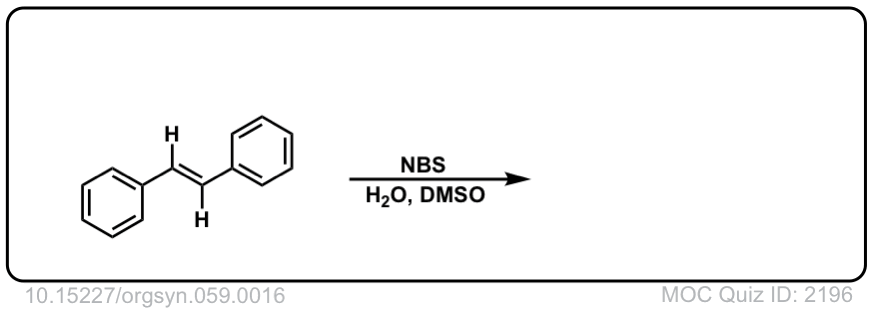 Click to Flip
Click to Flip
Org. Synth. 1977, 56, 112
DOI Link: 10.15227/orgsyn.056.0112
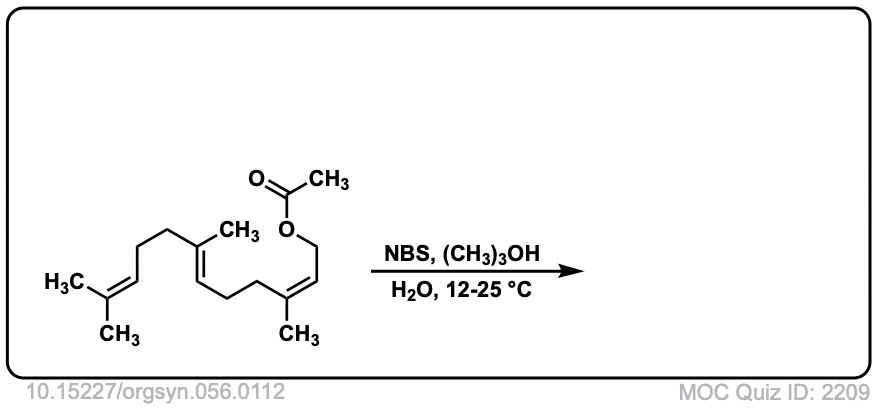 Click to Flip
Click to Flip
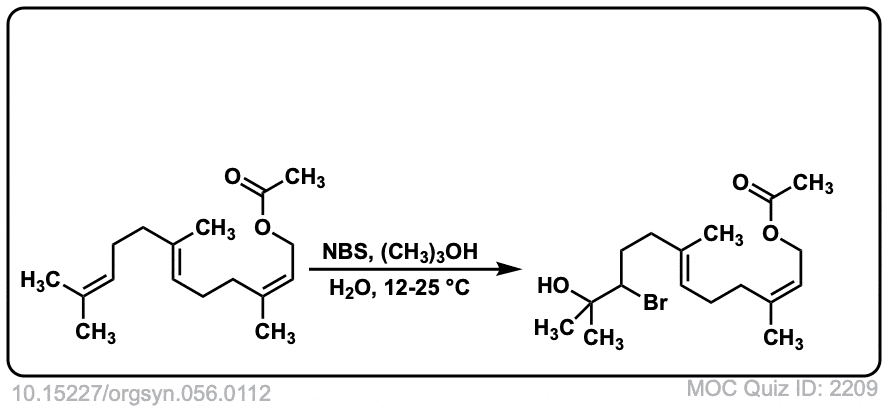
Why in the last example, Bromine isn’t in the wedge/dash position?
I was trying to show that the methyl groups on the cis double bond remain in the same position. I can see how it is not completely obvious that the stereochemisry of addition is trans. However, if you rotate the carbon on the left a little bit, it should be clear that the stereochemistry of the addition is still anti.
I ain’t good at chemistry….thnx to this website…. Btw in 2nd example why didn’t we attack other alkenes?… Is it due to low temp?
Are you referring to quiz 2209 at the bottom?
From the linked article (Org Syn)
“The oxidation procedure described above is intended to illustrate the selectivity that may be achieved using this system for functionalizing only one of several superficially similar double bonds in a molecule. In the case of acyclic terpenes, the yield of the desired terminal monobromohydrin decreases from 75–80% with geraniol (C10H18O) to 30–35% with squalene (C30H50), due to competing formation of polybromohydrins, allylic bromides, and bromocyclized material. The significant point, however, is that in all cases more than 95% of the monobromohydrin produced results from attack at the terminal double bond. A mechanistic investigation8 showed that the N-bromosuccinimide was not merely providing a source of bromine or hypobromous acid, and that the reaction was promoted by acid and inhibited by base.”
Thanks so much James, really fabulous website..
in second example, is there enantiomers?
Yes, the second example would have a mixture of enantiomers since there is a chiral center at C-2
in the mechanism, the lone pair attacks back to the double bond (right?) and if so, does it matter which side it attacks?
Yes it does. Thanks for bringing this up. Both bonds to the Br will be on the same face (formation of the bromonium ion is “syn”).
Is it possible to produce a halogenoalkane as a side product in this reaction, that is, Br- attacks the bromonium ion in the sec step?
yes, but keep in mind the concentration of solvent is significantly higher than the concentration of the counter-ion, so it will be very small [concentration of H2O in 1L of water is 55 M!] At very high concentrations it might become more significant.