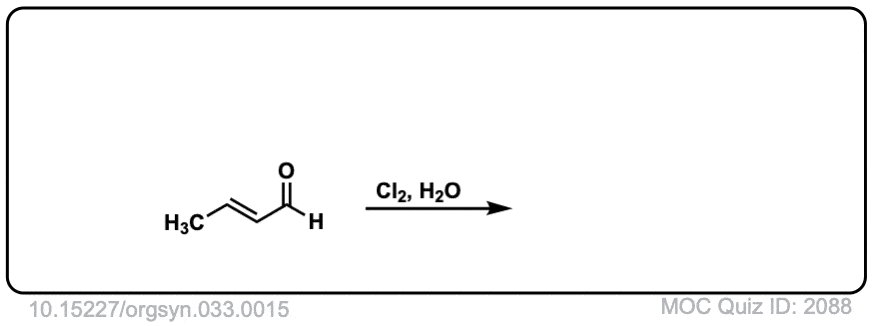
Formation of chlorohydrins from alkenes using water and Cl2
Description: Alkenes treated with chlorine (Cl2) in the presence of water will form chlorohydrins. The stereochemistry of the products is anti.
The rest of this page is available to MOC Members only.
To get access to this page, plus over 2500 quizzes, the Reaction Encyclopedia, Org 1 / Org 2 summary sheets, and flashcards, sign up here for only 30 cents/ day!
Real-Life Example:
Org. Synth. 1953, 33, 15
DOI Link: 10.15227/orgsyn.033.0015
 Click to Flip
Click to Flip
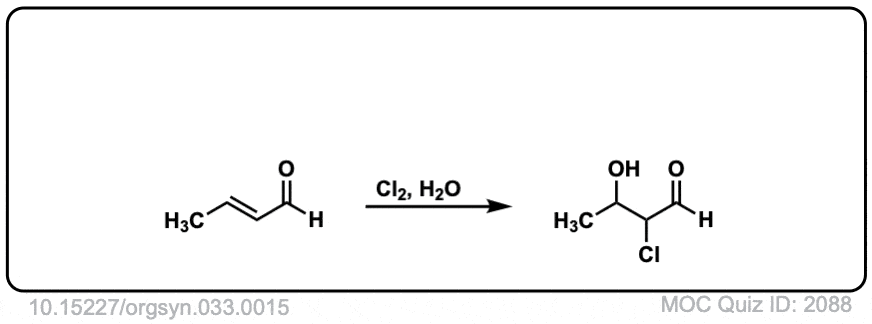
It’s may seem weird here that H2O is attacking the carbon further away from the C=O bond. A quick calculation (using Rowan) shows that the carbon adjacent to the carbonyl (C2) forms a stronger (and shorter) bond to Cl (1.86 Å) than the more distant C3-Cl bond length (1.97 Å). This is due to the strongly electron-withdrawing carbonyl group removing electron density from C-2, which results in a compensatory stronger C2-Cl bond.
The weaker C3-Cl bond is more likely to break.
MOC Quiz ID: 2088 – Why does the OH group attach further from the aldehyde group and Cl closer?
Similar to 2066, the presence of the carbonyl takes electron density away from the alpha carbon, which must result in a stronger C-X bond in the halonium ion to compensate.
The OH breaks the weaker bond of the halonium ion which in this case is the “less substituted” carbon. An interesting exception to Markovnikov’s rule, which can happen sometimes when electron-withdrawing groups are present.
In the step 2, why does H2O attack not chloride ion? isn’t chloride ion more nucleophile than water? Thanks.
Because water is solvent (or co-solvent) and vastly outnumbers the amount of chloride ion.
Hi!
In the 3rd reaction, why wouldn’t a 6 membered ring get formed?…like with chlorine on the ring?
Great question. In general formation of 5-membered rings is about 10 times faster than formation of 6-membered rings.
See: https://en.wikipedia.org/wiki/Baldwin%27s_rules#:~:text=Baldwin's%20rules%20in%20organic%20chemistry,closures%20of%20these%20various%20types.
hi , in the 3rd reaction , i checked the mechanism , based on what you choose the intra OH to attack instead of water ? thank you :)
Intramolecular reactions are generally faster than intermolecular reactions, especially if you’re forming a 5 or 6 membered ring.
In the third example, why doesn’t a six-member ring form?
Great question. In general, formation of 5-membered rings is about 10 times faster than formation of 6-membered rings.
https://en.wikipedia.org/wiki/Baldwin%27s_rules#:~:text=Baldwin's%20rules%20in%20organic%20chemistry,closures%20of%20these%20various%20types.
it is a *chloronium ion, not a bromonium ion, correct? just a typo, but worth fixing :)
yes, thank you! Fixed.
could you by any chance explain the mechanism of the 3 rxn? trying to get it and I’m not sure if the water that just attacked leaves or if the HCl adds an H to the other OH and then that leaves
Agree – the mechanism of the 3rd rxn is not clear
I drew a picture of the mechanism of this reaction, see here: http://imgur.com/ybbrTim
Hi,
according to the mechanism drawn, should the reaction also happen without water? Chloride can act as a base in that case.
Thank you for that, James.
Numbering the carbons will help
MarkovnikoV*
I use both misspellings of the name. : – )
what is the mechanism of the last reaction
The mechanism is exactly the same, except the oxygen is attached to the rest of the molecule.