Formation of epoxides from alkenes using m-CPBA
Description: meta-chloroperoxybenzoic acid (m-CPBA) or other peroxyacids such as peracetic acids will convert alkenes into epoxides.
The rest of this page is available to MOC Members only.
To get access to this page, plus over 2500 quizzes, the Reaction Encyclopedia, Org 1 / Org 2 summary sheets, and flashcards, sign up here for only 30 cents/ day!
Real-Life Examples:
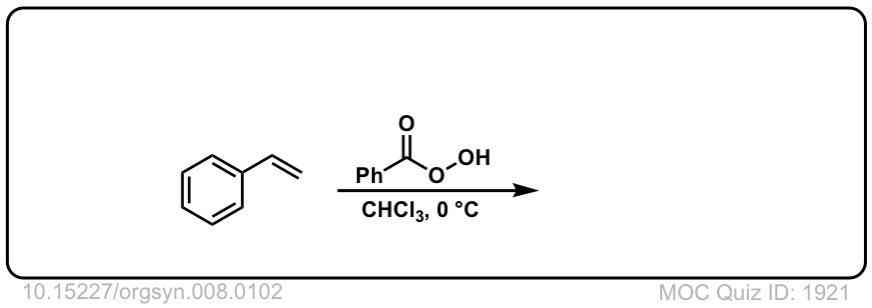
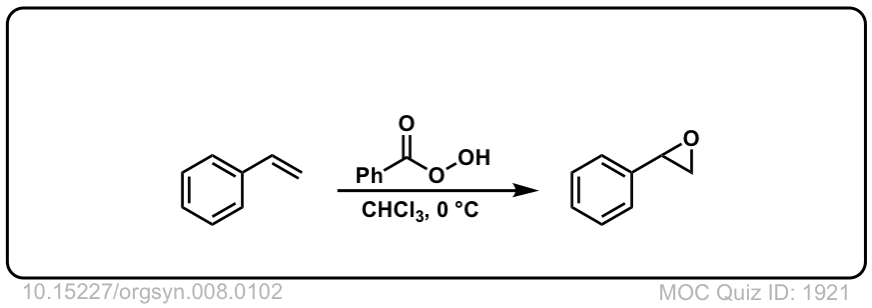
Org. Synth. 1928, 8, 102
DOI Link: 10.15227/orgsyn.008.0102
 Click to Flip
Click to Flip
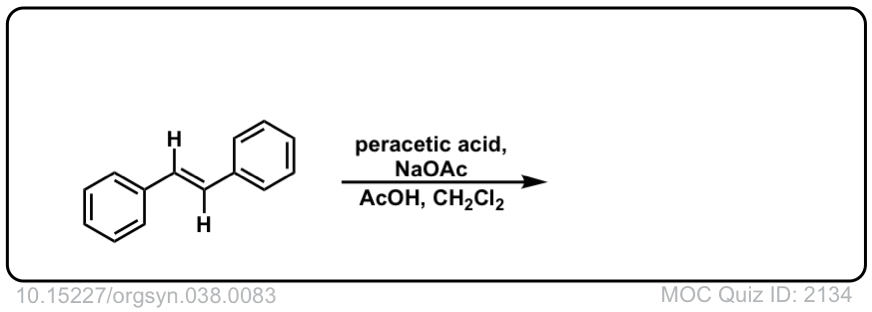
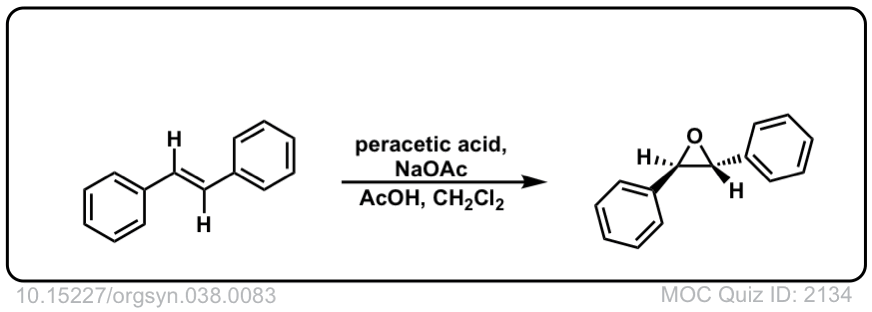
Org. Synth. 1958, 38, 83
DOI Link: 10.15227/orgsyn.038.0083
 Click to Flip
Click to Flip
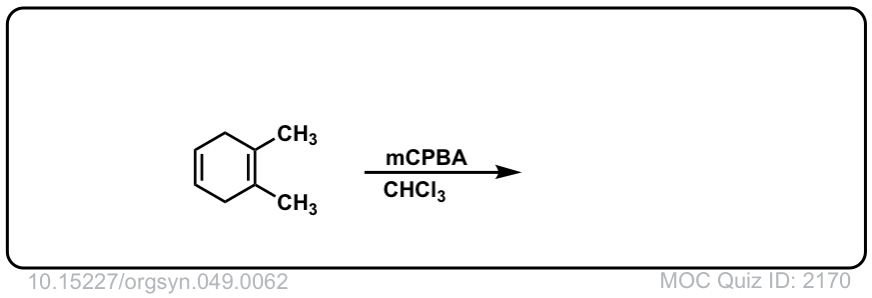
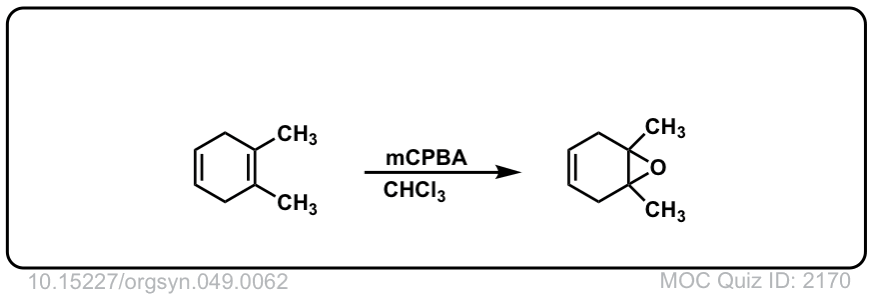
Org. Synth. 1969, 49, 62
DOI Link: 10.15227/orgsyn.049.0062
 Click to Flip
Click to Flip
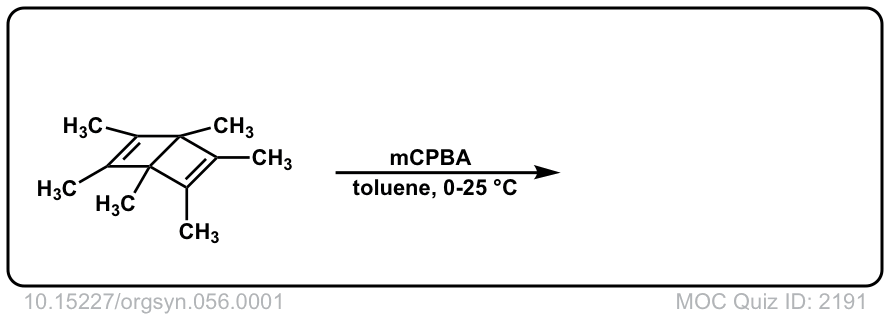
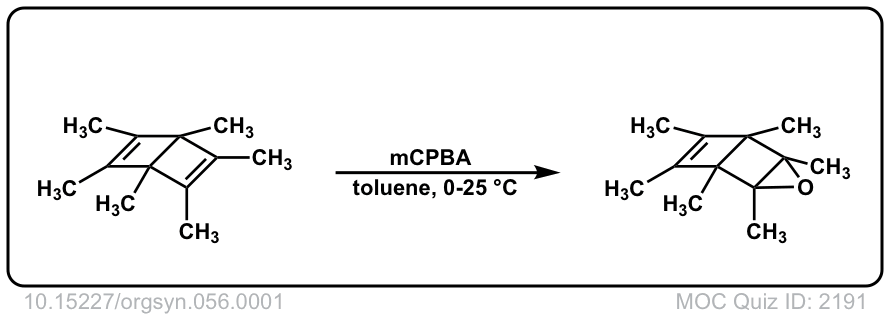
Org. Synth. 1977, 56, 1
DOI Link: 10.15227/orgsyn.056.0001
 Click to Flip
Click to Flip
MOC Quiz ID: 1618 is incorrect. The hydroxyl group should be on the more substituted carbon, as LiAlH4 opens the epoxide in a basic manner (via attack on the less substituted carbon). Is that correct?
You are correct. Fixed. Grateful to you for spotting this and bringing it to my attention.
Would this same reaction happen with DMDO/Acetone?
Yes! That’s another reagent for performing the same process.
What happens if you have a cyclohexane with two double bonds combined with two equivalents of mCPBA? Does it epoxidate both bonds?
It should. 1,4 cyclohexadiene or 1,3 cyclohexadiene? The di=epoxide from 1,4 cyclohexadiene is definitely known.
Thanks!
Will this same mechanism holds good even for the same reaction with Alkyne?
This reaction doesn’t work with alkynes! Can you think why not? [hint – it has something to do with aromaticity]
Has it got something to do with anti aromaticity?
Yes, alkynes do not react here, because they form anti-aromatic oxirenes (very unstable)
Thanks! ;)