Formation of Grignard Reagents from Alkyl Halides
Description: Treatment of alkyl halides with magnesium metal leads to formation of a Grignard reagent.
The rest of this page is available to MOC Members only.
To get access to this page, plus over 2500 quizzes, the Reaction Encyclopedia, Org 1 / Org 2 summary sheets, and flashcards, sign up here for only 30 cents/ day!
Real-Life Examples
DOI Link: 10.15227/orgsyn.006.0054
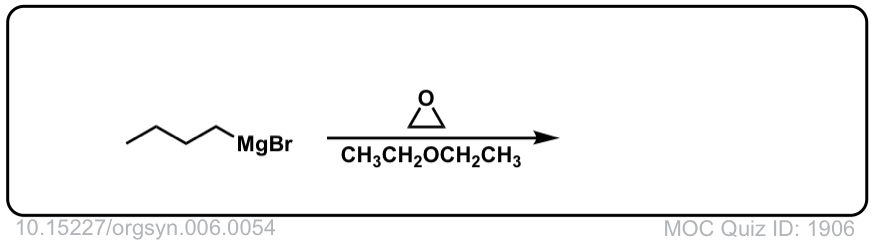 Click to Flip
Click to Flip
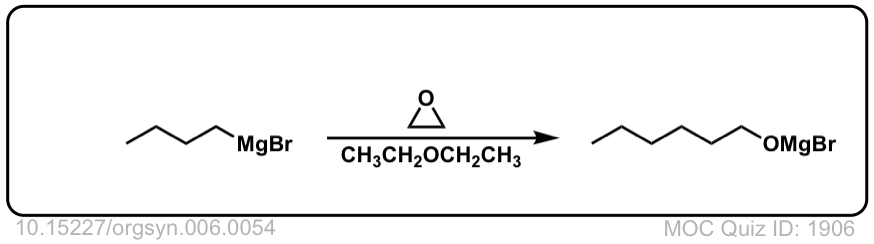
Org. Synth. 1930, 10, 4
DOI Link: 10.15227/orgsyn.010.0004
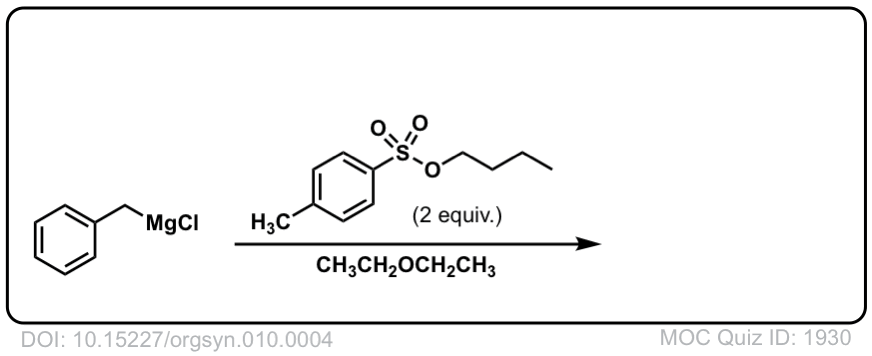 Click to Flip
Click to Flip
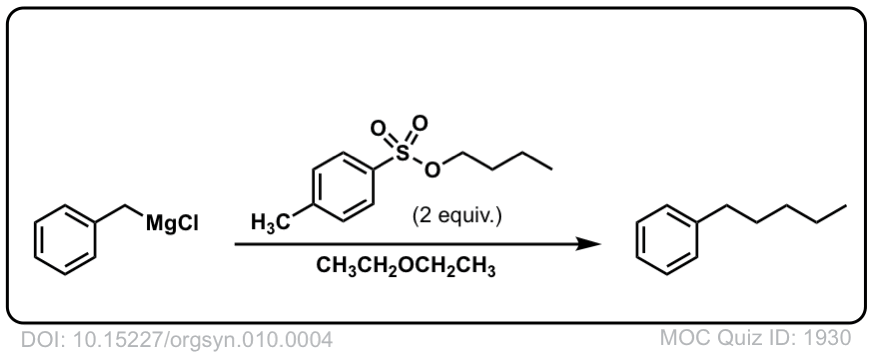
Org. Synth. 1931, 11, 66
DOI Link: 10.15227/orgsyn.011.0066
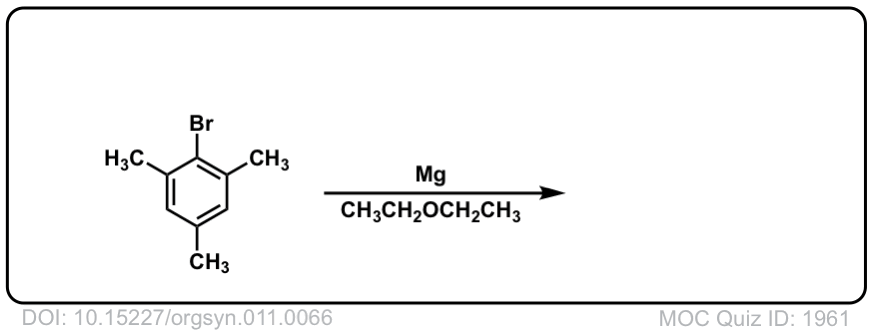 Click to Flip
Click to Flip
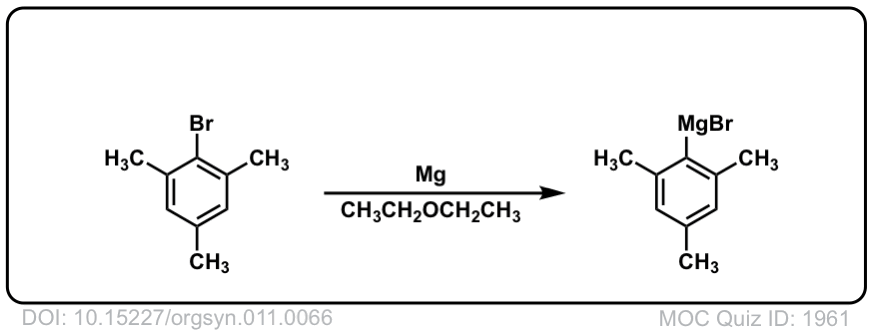
Org. Synth. 1932, 12, 48
DOI Link: 10.15227/orgsyn.012.0048
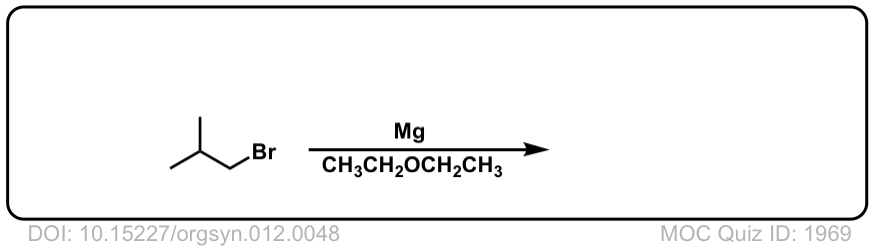 Click to Flip
Click to Flip
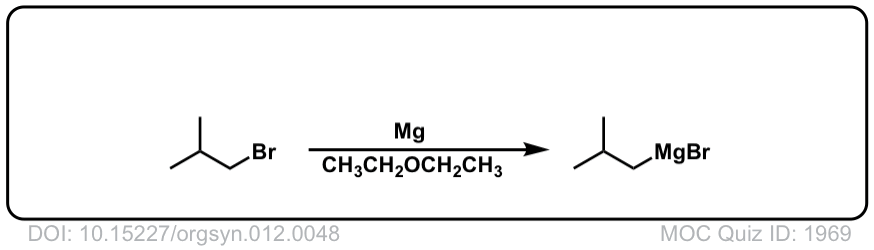
Org. Synth. 1931, 11, 80
DOI Link: 10.15227/orgsyn.011.0080
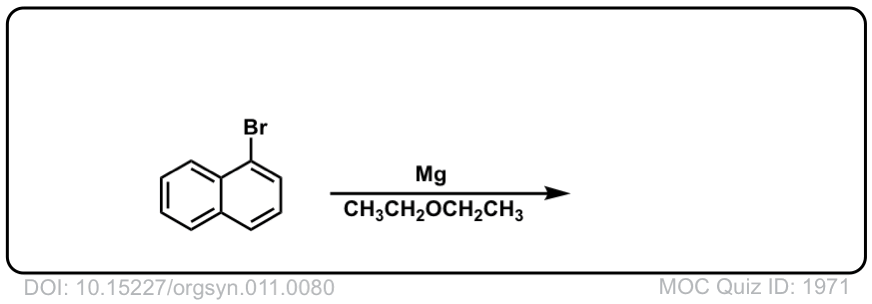 Click to Flip
Click to Flip
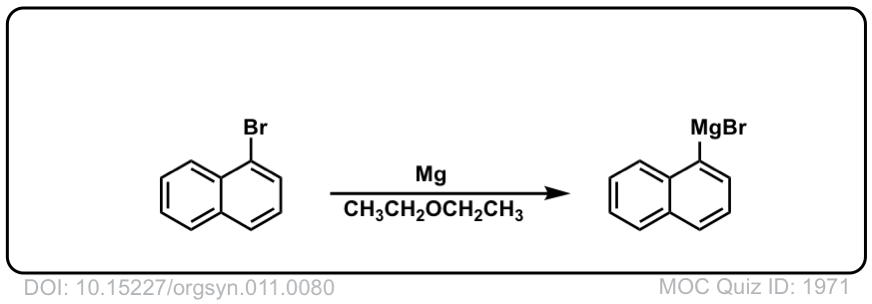
Org. Synth. 1931, 11, 84
DOI Link: 10.15227/orgsyn.011.0084
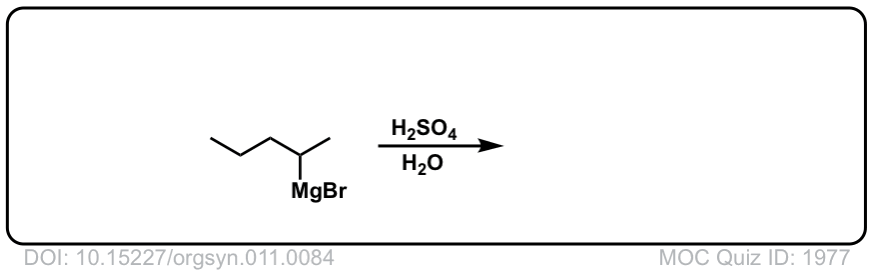 Click to Flip
Click to Flip
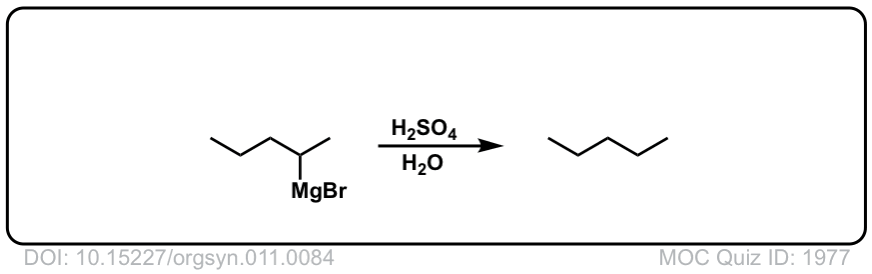
Org. Synth. 1947, 27, 65
DOI Link: 10.15227/orgsyn.027.0065
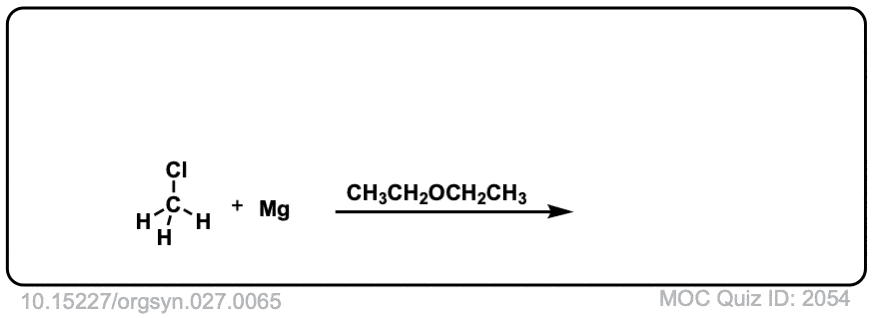 Click to Flip
Click to Flip
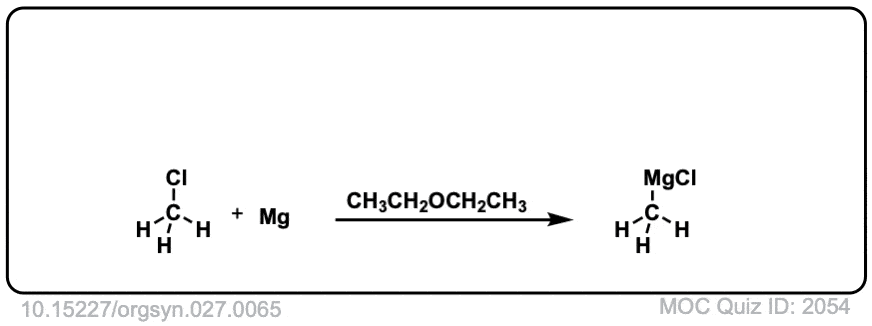
Good practice…
I don’t think I can view the image properly. The arrow pushing looks incorrect. But that’s okay. My book can finish this up, thank you.
OK. I agree that the image is blurrier than I’d like.
Would I be correct in stating that the Grignard reagent is so basic, with such a high pKa (approx. 50) that it is able to de-protonate any hydrogen with a lower pKa? Additionally, that in a synthesis it will de-protonate the most acidic hydrogen?