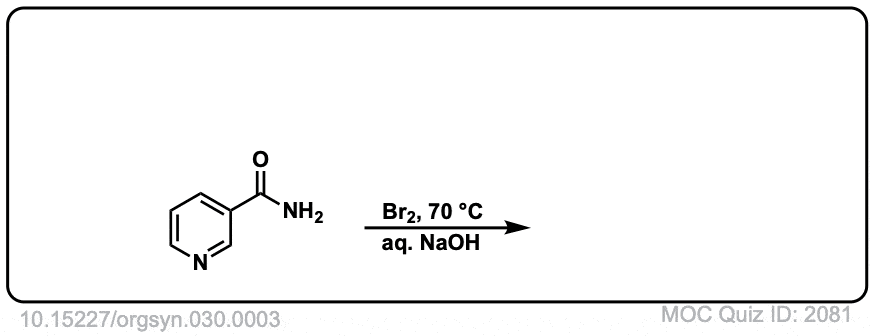
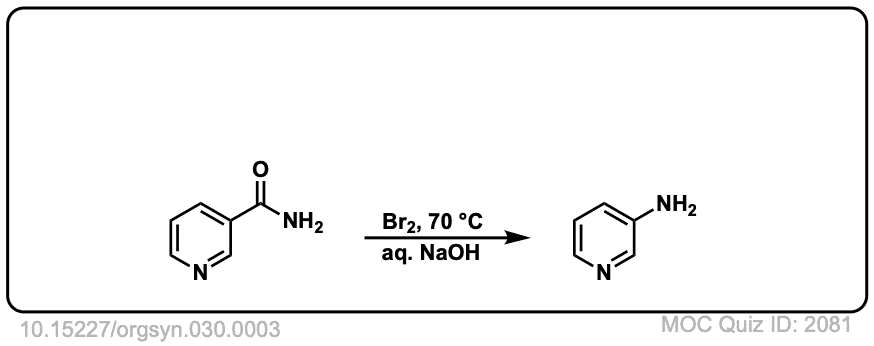
Hofmann Rearrangement of Amides to Amines
Description: When amides are treated with chlorine and base, a rearrangement occurs, resulting in the loss of carbon dioxide and the formation of an amine.
The rest of this page is available to MOC Members only.
To get access to this page, plus over 2500 quizzes, the Reaction Encyclopedia, Org 1 / Org 2 summary sheets, and flashcards, sign up here for only 30 cents/ day!
Real-Life Example:
Org. Synth. 1950, 30, 3
DOI Link: 10.15227/orgsyn.030.0003
 Click to Flip
Click to Flip
I’m wondering why the last example can react without amide. Is there something different happened? Or the carboxylic acid actually refers to amide?
I would appreciate for your help.
Sorry that was a typo. Fixed!
Why do you use bromine to get amines?
Would you be willing to post an example where Pyruvic acid (2-oxopropanoic acid) and N-hydroxymethanamine react under acidic conditions (H3O+) to form Peptin (N-methylacetamide)? The electron flow of this reaction is quite confusing.
Ah the Beckmann rearrangement. Very closely related! I should put it up here, but in the meantime… there’s always Wikipedia
How do the last two reactions in your examples work if there is no amide to start with? Where does the nitrogen come from?