Iodination of alkenes to give vicinal diiodides (1,2-diiodides)
Description: Treatment of alkenes with iodine (I2) leads to the formation of vicinal diiodides (1,2-diiodides).
The rest of this page is available to MOC Members only.
To get access to this page, plus over 2500 quizzes, the Reaction Encyclopedia, Org 1 / Org 2 summary sheets, and flashcards, sign up here for only 30 cents/ day!
Real-Life Example:
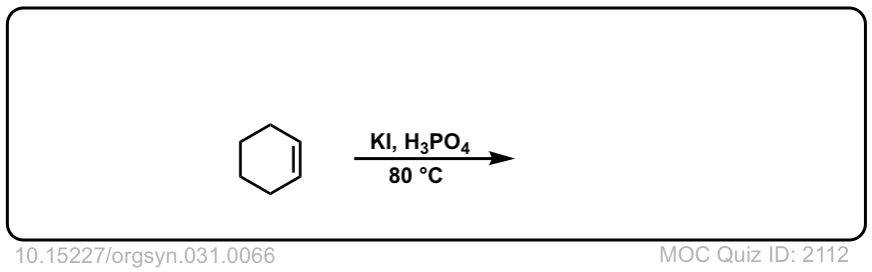
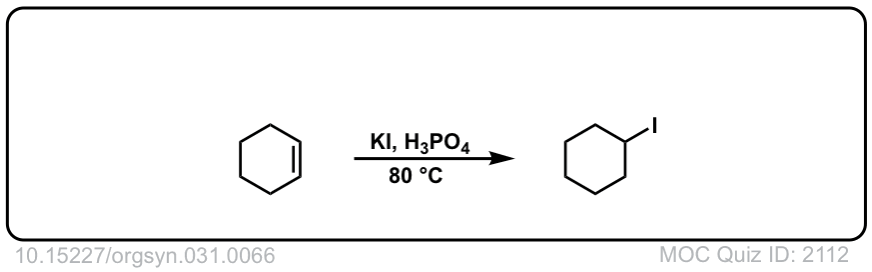
Org. Synth. 1951, 31, 66
DOI Link: 10.15227/orgsyn.031.0066
 Click to Flip
Click to Flip
Aren’t vicinal di-iodides unstable? Shouldn’t they give back the double bond (under what conditions is this reaction feasible)? Also, what is the mechanism for two vicinal di-iodides leaving.
Yes, they are quite unstable, especially to light.
The mechanism for di-iodides is the microscopic reverse of the forward reaction. Formation of an iodonium ion (with displacement of iodide) followed by attack of iodide on the iodonium to give back I2 and the double bond.
How is the 5th example a meso compound when there is no internal line of symmetry? one iodine is on a wedge while the other is on a dashed line.
I hate to nitpick little things like this (this resource is amazing I really appreciate it). I noticed the formation of the Iodonium ion in the main mechanism uses lines for the C-I connections, while in other examples a wedge was used
From one nitpicker to another, thank you. Easy thing to fix (done). Anything else, just let me know! James
Since this is anti-additon, in the fourth example, shouldn’t the I’s on the third and fourth Carbon’s be one wedged and one dashed not both wedged and dashed?
If you look closely the central carbon-carbon bond has also rotated. If that rotation had not happened, then the two C-I bonds would be anti.
This is a very common type of exam problem, where the question will start with an alkyne and then do either Lindlar or Na/NH3 to give a cis- or trans- alkene, and then do either a syn- or anti- selective reaction in the second step.
[wp_quiz_pro id="19026"]