Nucleophilic Aromatic Substitution Via Arynes
Description: Treatment of an aromatic containing a good leaving group with a strong base will lead to formation of an “aryne”. Attack of the aryne with a nucleophile then results in a product which is formally the result of nucleophilic aromatic substitution.
The rest of this page is available to MOC Members only.
To get access to this page, plus over 2500 quizzes, the Reaction Encyclopedia, Org 1 / Org 2 summary sheets, and flashcards, sign up here for only 30 cents/ day!
Real-Life Examples:
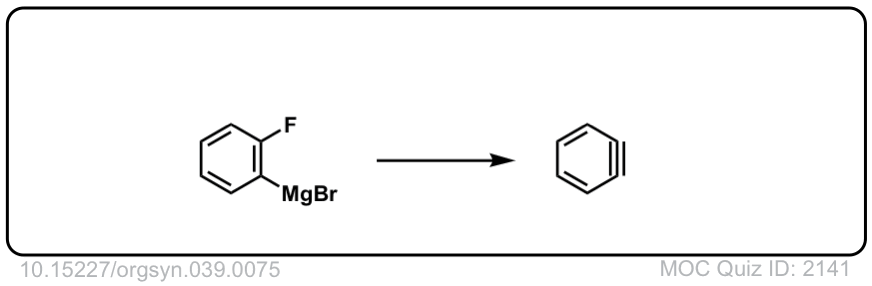
Org. Synth. 1959, 39, 75
DOI Link: 10.15227/orgsyn.039.0075
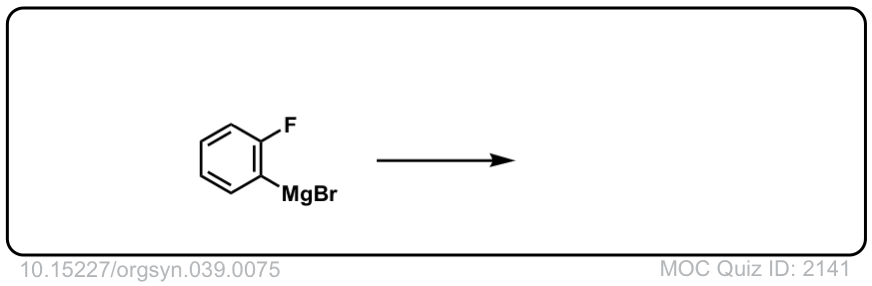 Click to Flip
Click to Flip
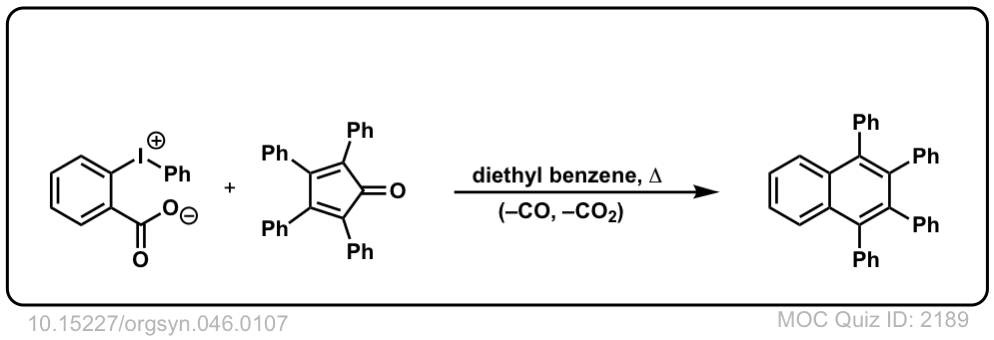
Org. Synth. 1966, 46, 107
DOI Link: 10.15227/orgsyn.046.0107
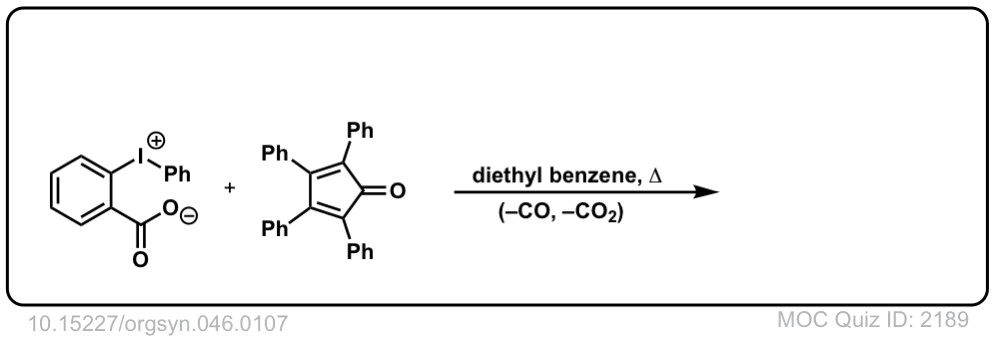 Click to Flip
Click to Flip
Nucleophilic aromatic substitution via arynes (a.k.a. benzynes) does not seem to require electron-withdrawing groups ortho or para to the leaving group in order to activate it–why is that?
The reason is that it goes through a different mechanism.
It’s an elimination reaction, essentially. The strong base (NaNH2) breaks C-H on the ring which then leads to elimination of an adjacent leaving group (such as Br or Cl) forming the pseudo triple bond.
None of the pi bonds in the aromatic ring are formed or broken.
In nucleophilic aromatic substitution, a nucleophile attacks the ring and forms a new bond, breaking one of the pi bonds in the ring. The resulting negative charge is then stabilized by one of the electron withdrawing groups. Then the leaving group is expelled with restoration of aromaticity.
Shorter version: in arene formation, aromaticity is never disrupted.
Hope this helps! James
In the mechanism, the last entry in the “bond broken” list may require some attention.