Olefin Metathesis
Description: Treatment of alkenes with Grubbs’ catalyst (pictured; “Ru” is ruthenium) results in a “swapping” of the terminal carbons of alkenes, a reaction known as olefin metathesis.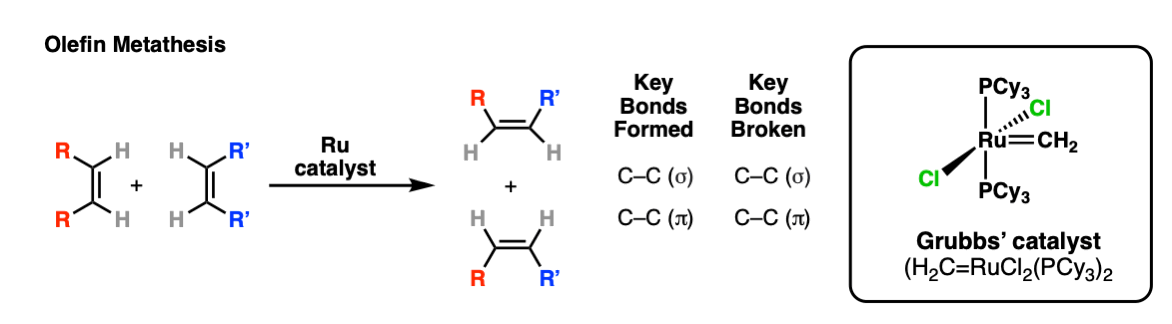
Notes: The most effective version of this reaction is “ring-closing metathesis”, where two terminal alkenes are joined together to form a new ring.
Examples:
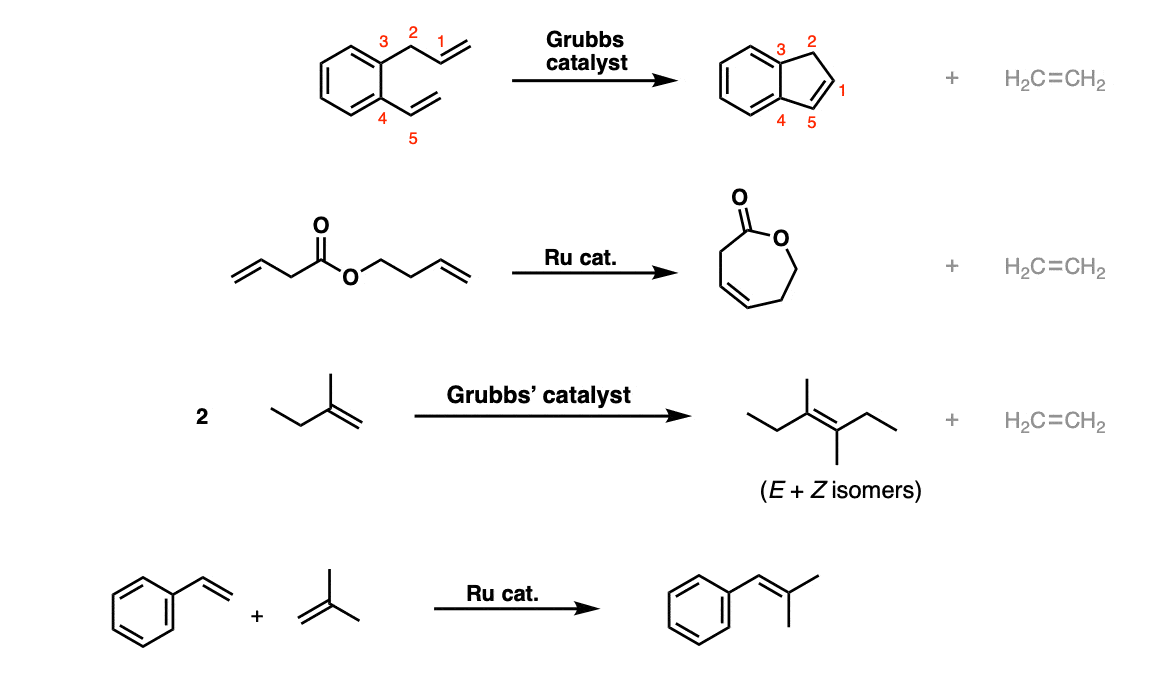
Notes: The first and second examples show typical “ring-closing” metathesis reactions (an intramolecular reaction) where the the interior carbons on each alkene form a ring, and the terminal CH2 groups on each alkene come together to form CH2=CH2 . The third and fourth reactions join together two alkenes in “intermolecular” fashion, a variant called “cross-methathesis”. The reaction works best when one of the alkenes is present in excess.
Instead of drawing out Grubbs’ catalyst, just writing “Grubbs catalyst” or “Ru catalyst” is commonly done.
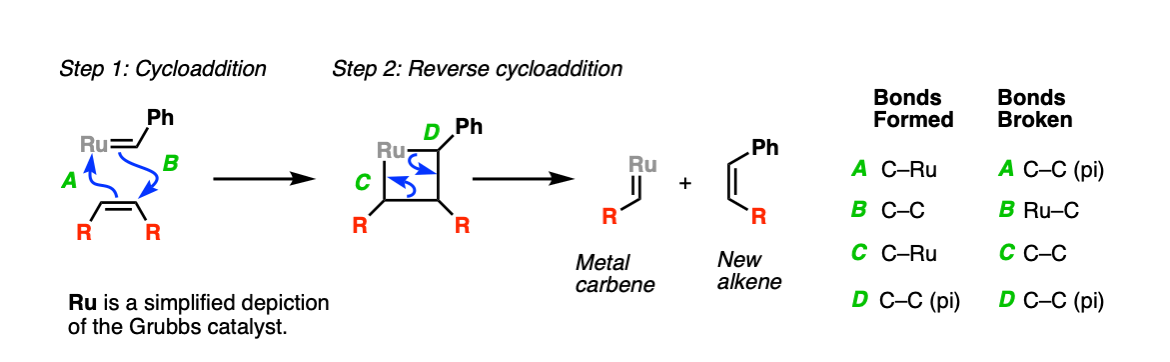
Mechanism:
The first step is a cycloaddition between the alkene and the ruthenium carbene to form a 4-membered ring (Step 1, arrows A and B). After this occurs, the second step is a reverse cycloaddition (Step 2, arrows C and D) to give a new ruthenium carbene and a new alkene (olefin).
Note that to keep things simple the other groups attached to the Grubbs’ catalyst (“ligands”) are omitted, and “Ru” is drawn.
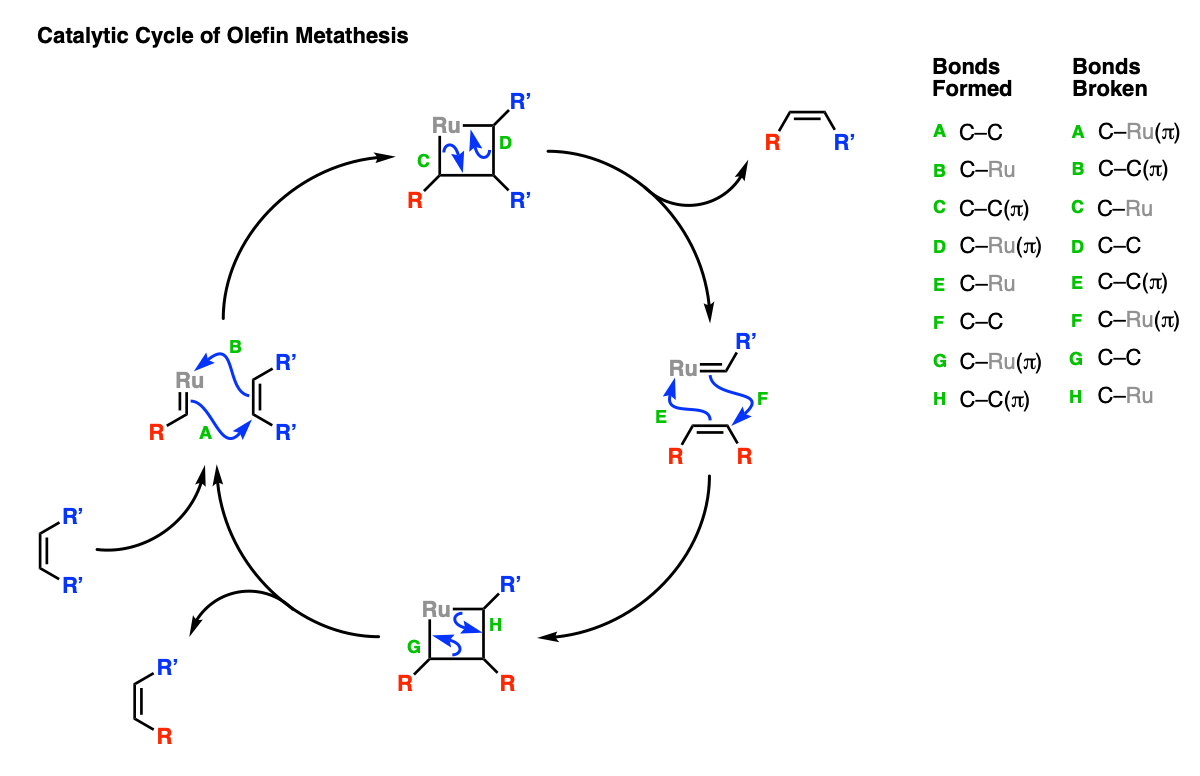
The process is catalytic. Here is the catalytic cycle.
Step 1 (arrows A and B) shows the initial cycloaddition between the ruthenium carbene and the alkene. The reverse cycloaddition (Step 2, arrows C and D) results in a new alkene as well as a new ruthenium carbene. The carbene can then combine with a new equivalent of alkene (Step 3, arrows E and F) followed by another reverse cycloaddition (Step 4, arrows G and H).
Notes: The geometry of the alkene is not very well-controlled in the intermolecular version of this reaction (“cross-metathesis”), so a mixture of E and Z alkenes will usually be obtained.