Oxidation of aldehydes to carboxylic acids with Ag2O
Description: When Ag2O is added to aldehydes, carboxylic acids are formed. This results in the formation of silver metal (“silver mirror”) a reaction known as the Tollens test.
The rest of this page is available to MOC Members only.
To get access to this page, plus over 2500 quizzes, the Reaction Encyclopedia, Org 1 / Org 2 summary sheets, and flashcards, sign up here for only 30 cents/ day!
Real-Life Examples:
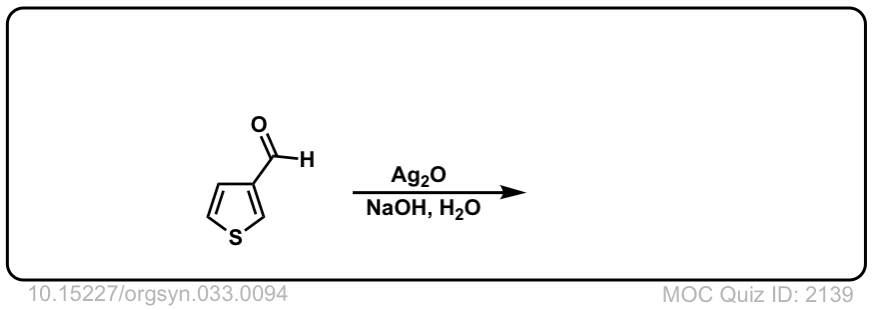
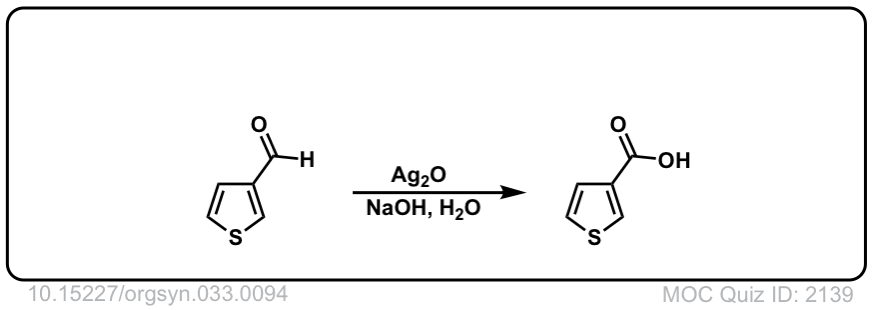
Org. Synth. 1953, 33, 94
DOI Link: 10.15227/orgsyn.033.0094
 Click to Flip
Click to Flip
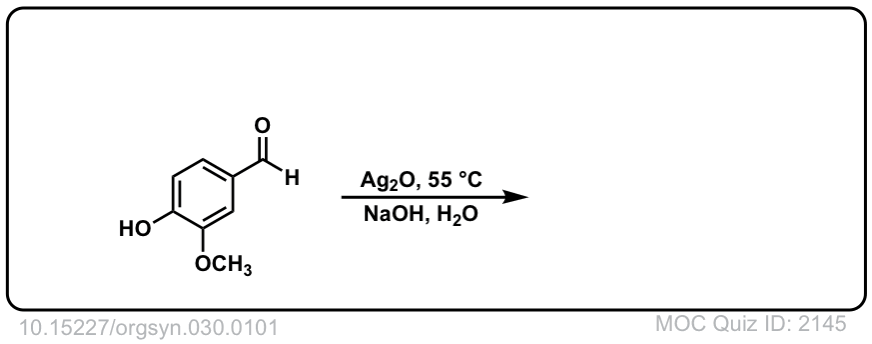
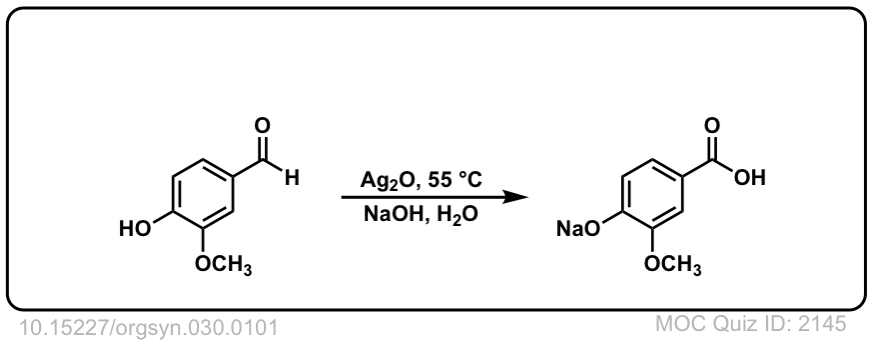
Org. Synth. 1950, 30, 101
DOI Link: 10.15227/orgsyn.030.0101
 Click to Flip
Click to Flip
Will ketones undergo oxidation with Ag2O?
No, only aldehydes. The only way ketones will undergo oxidation is with a reagent like mCPBA (doing the Baeyer-Villiger) or with something like KMnO4
Could this also be done with other oxidation agents?
Sure you can use any other mild or strong oxidising agents like Fehling Solution, HNO3,acidic/basic KMnO4/K2Cr2O7 and many more..
pretty sure acidified Potassium DiChromate does the same thing :)
Is this reagent the same as Ag(NH3)2+?
The oxidation state of Ag+ is the same, and there will be some similar properties, but they are not the same thing. Ag(NH3)2+ is much better at forming complexes with alkenes, for example.