Reduction of aromatic nitro groups to amino groups
Description: Nitro groups can be converted into amino groups by treatment with a reducing agent such as palladium on carbon with hydrogen gas (Pd/C, H2), zinc metal with acid, or tin (Sn) with acid.
The rest of this page is available to MOC Members only.
To get access to this page, plus over 2500 quizzes, the Reaction Encyclopedia, Org 1 / Org 2 summary sheets, and flashcards, sign up here for only 30 cents/ day!
Real-Life Examples:
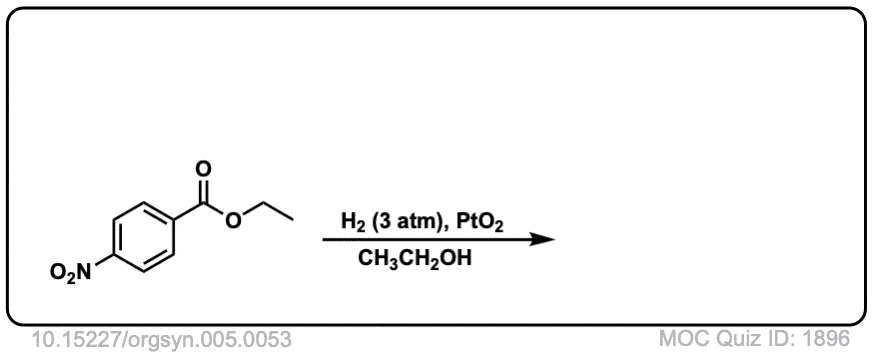
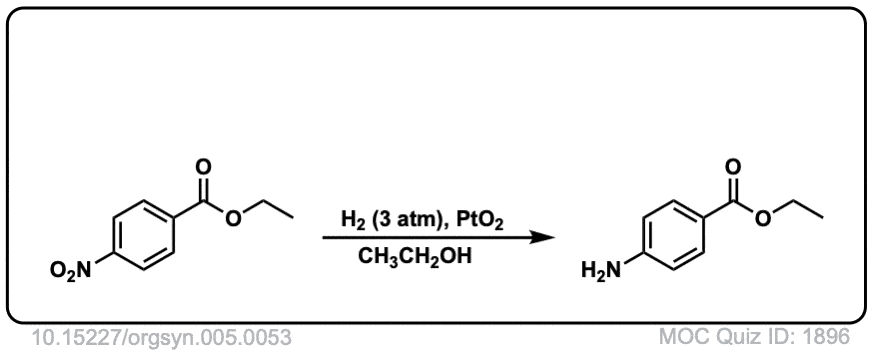
Org. Synth. 1928, 8, 66
DOI Link: 10.15227/orgsyn.5.0053
 Click to Flip
Click to Flip
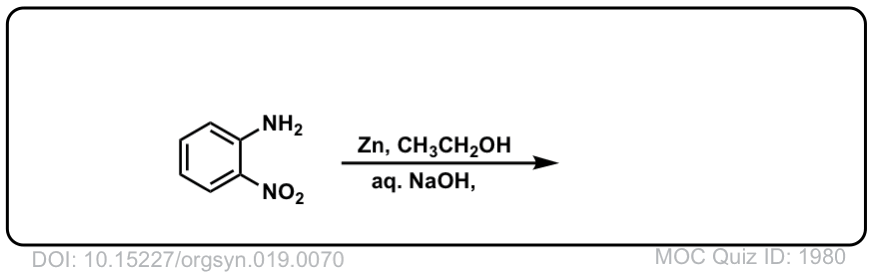
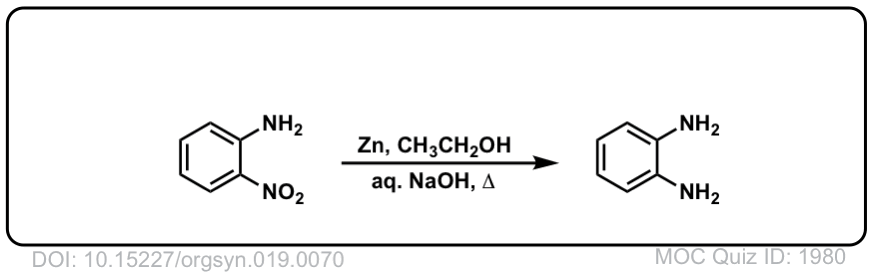
Org. Synth. 1939, 19, 70
DOI Link: 10.15227/orgsyn.019.0070
 Click to Flip
Click to Flip
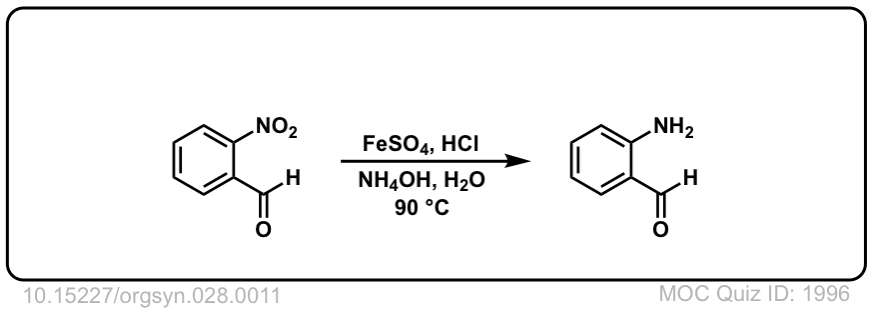
Org. Synth. 1948, 28, 11
DOI Link: 10.15227/orgsyn.028.0011
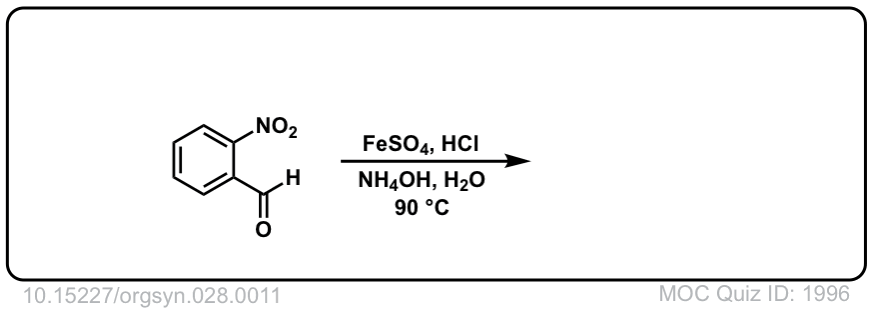 Click to Flip
Click to Flip
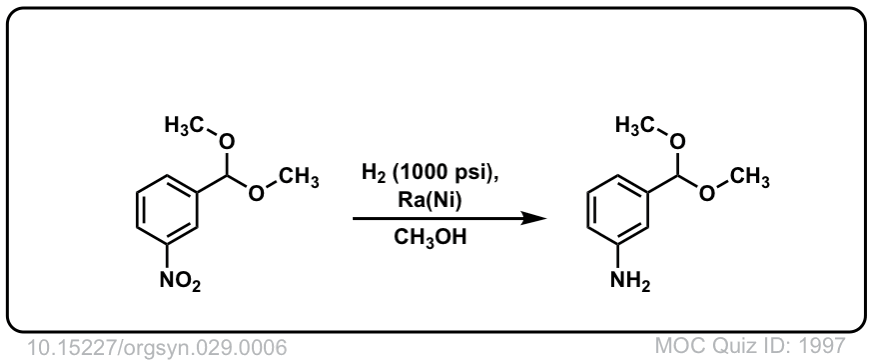
Org. Synth. 1949, 29, 6
DOI Link: 10.15227/orgsyn.029.0006
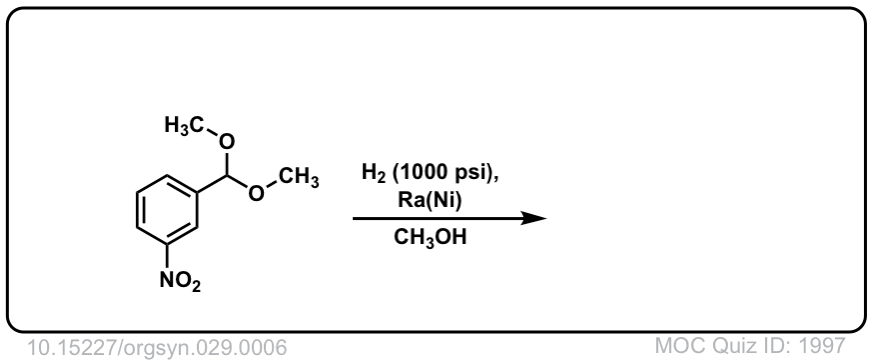 Click to Flip
Click to Flip
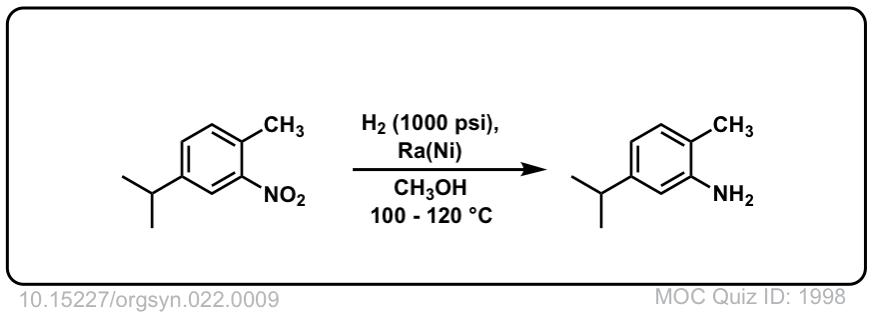
Org. Synth. 1942, 22, 9
DOI Link: 10.15227/orgsyn.022.0009
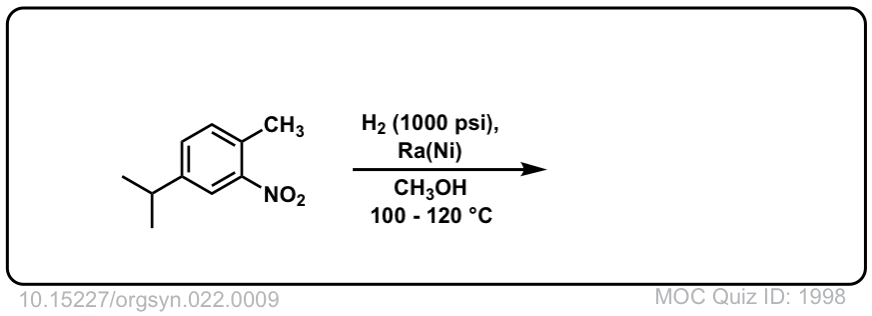 Click to Flip
Click to Flip
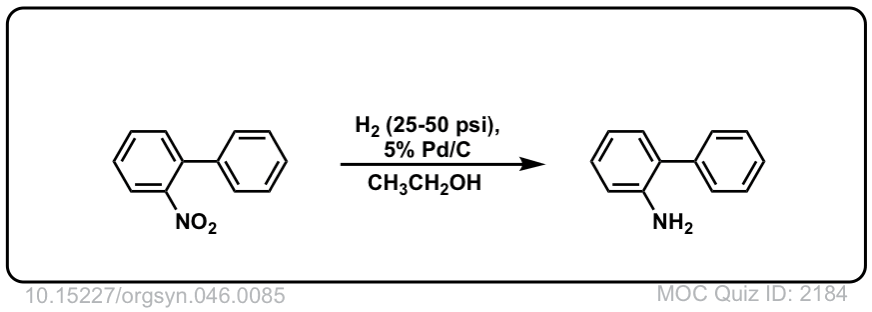
Org. Synth. 1966, 46, 85
DOI Link: 10.15227/orgsyn.046.0085
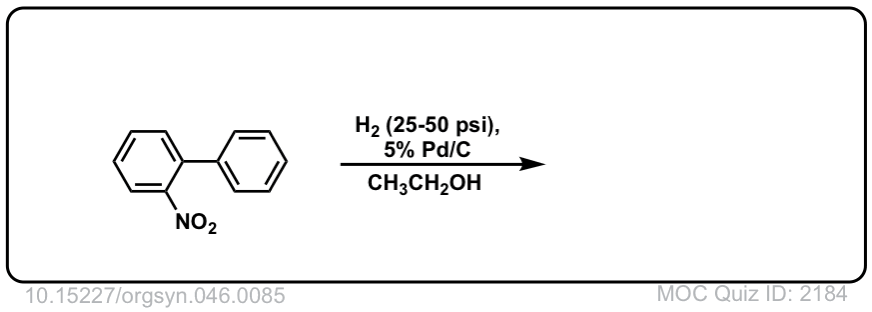 Click to Flip
Click to Flip
In converting the moderately activating -NHCOCH3 group to the stronger -NH, are there certain reaction conditions where H+/water are preferred over-OH/water? Or are they essentially interchangeable?
If the amide is the only functional group present on your molecule, it shouldn’t really matter (on paper, anyway!).
The only thing to watch out for is if you have a functional group that might be affected by strong base or strong acid. That generally doesn’t come up in introductory courses, but it can be a factor if you’re planning a synthesis. Strong aqueous acid, for example, will also hydrolyze an ester to a carboxylic acid; also, if you use acid, you have to specify a neutralization step with base, otherwise you will end up with an NH3+ group on the aromatic ring, which is a strong deactivating group.
Strong base will likewise hydrolyze any esters you might have, as well as potentially react (e.g. SN2) with alkyl halides if they are present.
Those are some of the major things to watch out for. Again, for most introductory courses, probably doesn’t matter too much.
Would an iron based reagent work as well and do you need to use NaOH for the second step in the reagents with acids?