Reductive Amination
Description: Aldehydes and ketones can be converted into amines, through formation of an imine and treatment with a reducing agent. This is called “reductive amination”.
The rest of this page is available to MOC Members only.
To get access to this page, plus over 2500 quizzes, the Reaction Encyclopedia, Org 1 / Org 2 summary sheets, and flashcards, sign up here for only 30 cents/ day!
Real-Life Examples:
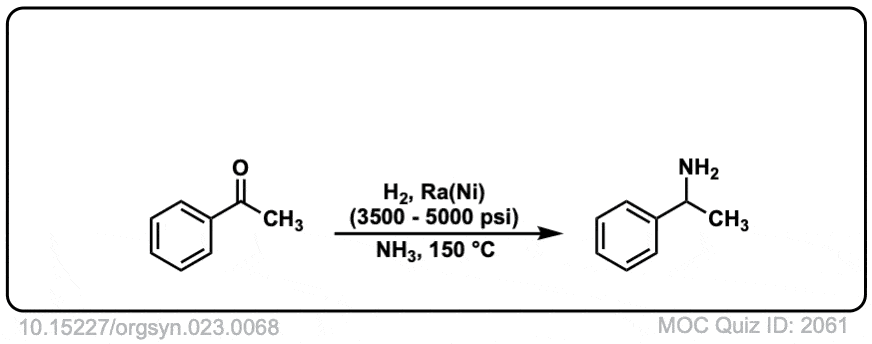
Org. Synth. 1943, 23, 68
DOI Link: 10.15227/orgsyn.023.0068
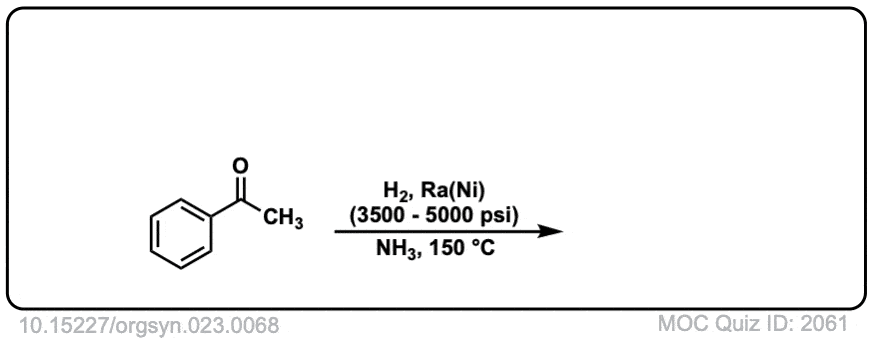 Click to Flip
Click to Flip
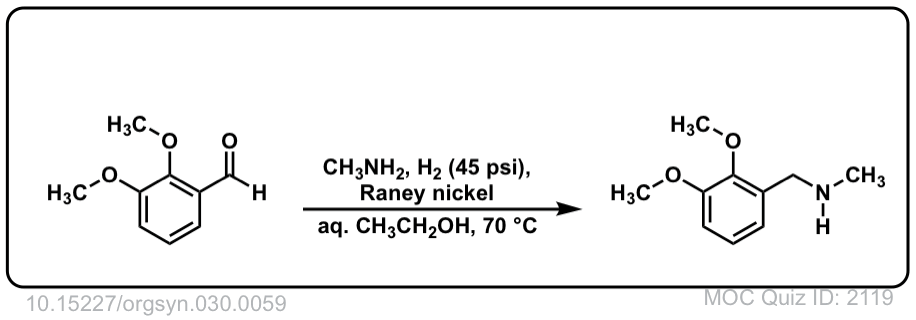
Org. Synth. 1950, 30, 59
DOI Link: 10.15227/orgsyn.030.0059
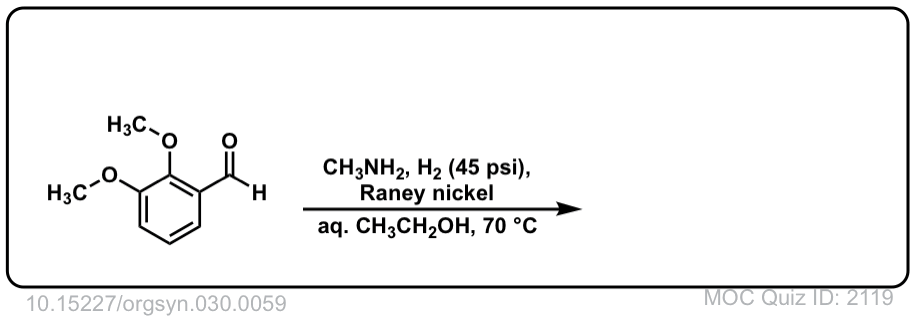 Click to Flip
Click to Flip
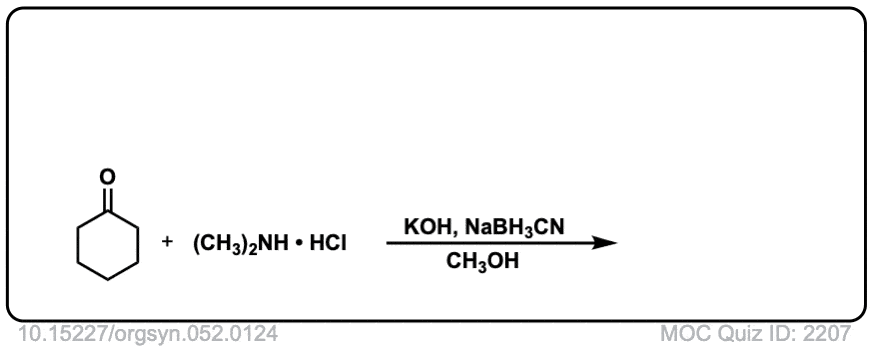
strong>Org. Synth. 1972, 52, 124
DOI Link: 10.15227/orgsyn.052.0124
 Click to Flip
Click to Flip
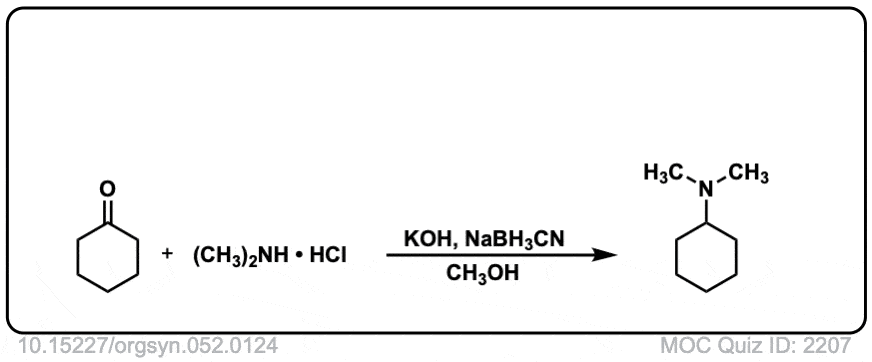
Shouldn’t the finished product in quiz 1820 lead to a tertiary amine, rather than a secondary amine?
Yes. You are correct. Putting this in my fix queue, will get it done today or tomorrow. Thank you! [Edit: fixed]
Why does the Reducing agent attack carbon instead of deprotonating Nitrogen to form H2 gas?
For the quiz #1820, why there’re not amines with two methyl groups but with one hydrogen instead ?
(According to the mechanism, only the hydrogen on the amine would be eliminated in the proton transfer step.)
I think that maybe I have missed something crucial, so I have hard time understanding the product of quiz #1820.
Thank you very much.
This has been fixed. Thank you!
The finished product in the mechanism you are showing has an additional carbon that it should not have. To the right of the red H in the finished product should be an additional hydrogen.
Fixed. Thank you very much!